This 2021 rodent study investigated post-traumatic stress disorder (PTSD) susceptibility:
“PTSD is an incapacitating trauma-related disorder, with no reliable therapy. We show distinct DNA methylation profiles of PTSD susceptibility in the nucleus accumbens (NAc). Data analysis revealed overall hypomethylation of different genomic CpG sites in susceptible animals.
Is it possible to treat PTSD by targeting epigenetic processes? Such an approach might reverse genomic underpinning of PTSD and serve as a cure.
To test plausibility of such an approach, a reliable animal (rat) model with high construct validity is needed. Previously, we reported one such model, which uses predator-associated trauma, and cue reminders to evoke recurring trauma. This simulates clinical PTSD symptoms including re-experiencing, avoidance, and hyperarousal.
Individual PTSD-like (susceptible) behavior is analyzed, enabling identification of susceptible animals separately from those that are non-PTSD-like (resilient). This model captures salient features of this disorder in humans, in which only a fraction of trauma victims develop PTSD, while others are resilient.

Sprague–Dawley rats were exposed to trauma and to three subsequent trauma-associated reminders. Freezing behavior was measured under conditions of:
- Exploration;
- Social interaction (with a companion); and
- Hyperarousal.
Controls were exposed to identical conditions except for the traumatic event.
PTSD-like behavior of each animal was compared with baseline and with the population. Two unambiguous sub-populations were identified, resilient and susceptible.
After exposure to trauma and its reminders, susceptible animals showed an increase from baseline in freezing behavior, and over time in all three behavioral tests, as opposed to resilient and control groups.
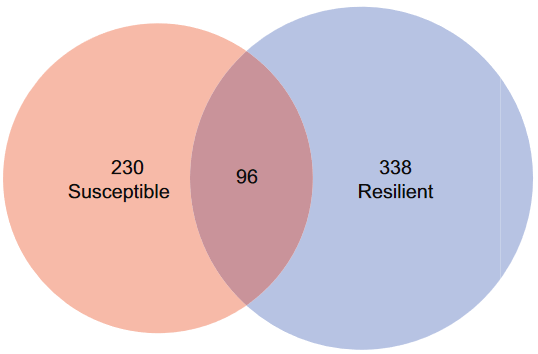
Differentially methylated sites in susceptible and resilient animals compared to control group.
Although we focused in this study on DNA methylation changes that associate with susceptibility, we also report unique changes in DNA methylation that occur in resilient animals. Inhibition of critical genes that are downregulated in susceptible animals convert resilient animals to become susceptible.”
https://www.researchgate.net/publication/353192082_Reduction_of_DNMT3a_and_RORA_in_the_nucleus_accumbens_plays_a_causal_role_in_post-traumatic_stress_disorder-like_behavior_reversal_by_combinatorial_epigenetic_therapy “Reduction of DNMT3a and RORA in the nucleus accumbens plays a causal role in post-traumatic stress disorder-like behavior: reversal by combinatorial epigenetic therapy” (registration required)
Rodents with the same genetics and environment displayed individual differences in their responses to traumatic events. Researchers, provide evidence for that before venturing elsewhere.
Not sure why it took 3+ years for this study received in November 2017 to finally be published in July 2021. Sites other than https://doi.org/10.1038/s41380-021-01178-y are more transparent about their peer review and publication processes.
No causes for PTSD susceptibility were investigated. PTSD effects and symptoms aren’t causes, notwithstanding this study’s finding that:
“Our results support a causal role for the NAc as a critical brain region for expression of PTSD-like behaviors, and a role for programming genes by DNA methylation in the NAc in development of PTSD-like behaviors.”
Can’t say that I understand more about causes for PTSD susceptibility now than before I read this study. Researchers attaching significance to gene functional groups seemed like hypothesis-seeking efforts to overcome limited findings.
Will this study’s combination of a methyl donor with a Vitamin A metabolite address PTSD causes in humans? If it only temporarily alleviates symptoms, what lasting value will it have?
Several brain and body areas that store traumatic memories other than the nucleus accumbens were mentioned in The role of recall neurons in traumatic memories. A wide range of epigenetic memory storage vehicles is one reason why effective human therapies need to address each individual, their whole body, and their entire history.

Osprey breakfast



























 Inauguration day
Inauguration day