Two reviews of Nrf2 relationships with our two immune systems, starting with adaptive immunity:
“We highlight recent findings about the influence of Keap1 and Nrf2 in development and effector functions of adaptive immune cells, T lymphocytes and B lymphocytes. We summarize Nrf2 research potential and targetability for treating immune pathologies.
Immune cells have mechanisms in place to strike a perfect redox balance, and to modulate levels of ROS differentially during their naive, activated, and effector stages for tailored immune responses. Cells of the lymphoid lineage (T, B, and NK cells) and myeloid lineage (macrophages, granulocytes, dendritic cells, and myeloid-derived suppressor cells) are generated from self-renewing progenitors, hematopoietic stem cell (HSCs) in the bone marrow.
Nrf2 activation in HSCs skews hematopoietic differentiation toward the myeloid lineage at the cost of the lymphoid lineage cells. Nrf2 does not participate in late T cell development leading to generation of single-positive CD4 and CD8 T cells.

- Nrf2 activation supports differentiation of the Th2 subset, regulatory T cells (Tregs), and the NKT2 subset while inhibiting differentiation of Th1, Th17, NKT1, and NKT17 subsets.
- The absence of or low Nrf2 results in enhanced proinflammatory responses, characterized by differentiation of Th1, Th17, NKT1, and NKT17 subsets, and subdued generation of Th2, Treg, and NKT2 subsets.
Nrf2 activation levels also influence generation of humoral responses.
- Low Nrf2 levels favor T cell–dependent production of IgG and IgM Abs by activated B cells.
- High Nrf2 suppresses B cell responses such as differentiation of germinal center B cells and plasma cells.
Nrf2 negatively regulates T–cell mediated inflammatory responses and T-dependent B cell responses.“
https://journals.aai.org/immunohorizons/article/7/4/288/263657/Beyond-Antioxidation-Keap1-Nrf2-in-the-Development “Beyond Antioxidation: Keap1–Nrf2 in the Development and Effector Functions of Adaptive Immune Cells”
And our innate immune system:
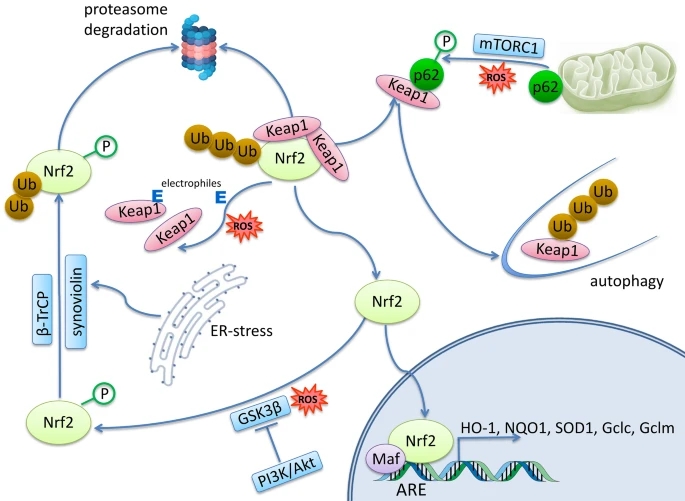
“Nrf2 regulates the immune response by interacting directly or indirectly with one or more of the major innate immune signaling components that maintain cellular homeostasis. Toll-like receptors (TLR) signaling can induce Nrf2 activation, and this is primarily found to be through autophagy-mediated degradation of Keap1.
TLR agonists may be considered as stimuli that induce Nrf2 to reduce stress and inflammation, linking the immune and antioxidant pathways. Conversely, Nrf2 activation may restrain TLR-mediated inflammatory response through induction of antioxidant proteins and inhibition of pro-inflammatory cytokines.
Following LPS stimulation, the NF-κB pathway is engaged to initiate a host of pro-inflammatory responses such as IL-6 and interleukin 1 beta (IL-1β) gene expression. Nrf2 induction inhibits LPS-mediated activation of pro-inflammatory cytokines in macrophages.
Inflammasome activation is an essential component of the innate immune response, and is critical for clearance of pathogens or damaged cells through pro-inflammatory cytokine secretion and/or cell-death induction. While Nrf2 activation is in general associated with an anti-inflammatory state, Nrf2 has also been reported to be required for optimal NLRP3 inflammasome activity.
The type-I interferon (IFN) system constitutes an essential part of innate immunity. Type-I IFNs are produced upon recognition of foreign or self-DNA or RNA, and are best-known for inducing an antiviral state through the induction of interferon-stimulated genes. While Nrf2 interferes with IRF3 activation, STING expression, and type-I IFN signaling, none of these crucial players in innate immunity have been demonstrated to be direct targets of Nrf2.
The antiviral effect of Nrf2 activation by 4-OI may use various pathways to limit viral replication that have not been identified yet. It is important to consider that Nrf2-activating metabolites may also act as immunomodulators in a Nrf2-independent manner.
Anti-inflammatory properties of Nrf2 are independent of redox control. Further mechanistic studies are needed to decipher the exact indirect and/or direct interactions between Nrf2 and innate immune players.”
https://www.sciencedirect.com/science/article/pii/S0952791522000942 “Regulation of innate immunity by Nrf2”