Four 2023 papers with different chondroitin sulfate doses, starting with a review:
“This article aims to provide an up-to-date view of current literature regarding biological effects and efficacy of chondroitin sulfate (CS), and discusses the quality of available CS supplements and the current direction in CS investigation.
A meta-analysis published in 2019 concluded that CS had small to moderate effectiveness in reducing osteoarthritis (OA)-related pain, with larger dosages (1200 mg/d) having greater benefits than smaller dosages. This meta-analysis concluded that CS had only a minimal effect on joint space narrowing and no effect on cartilage volume.
Chondroitin sulfate effects on osteoarticular tissues
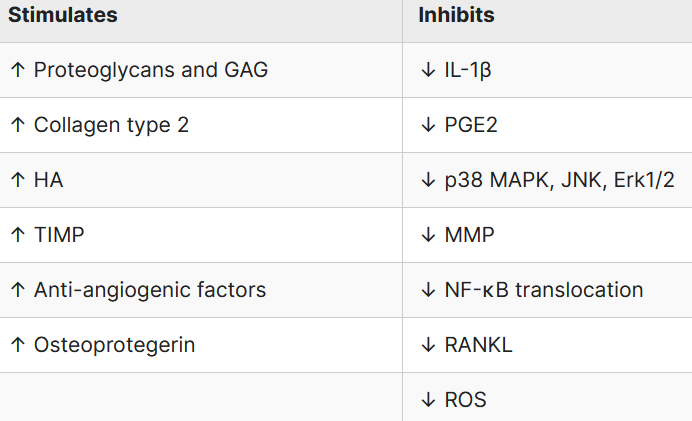
GAG: glycosaminoaglycans; HA: hyalouronic acid; TIMP: tissue inhibitor of metalloproteinase; MMP: metalloproteinase; ROS: reactive oxygen species; RANKL: receptor activator of nuclear factor kappa-Β ligand; JNK: c-Jun N-terminal kinase; PGE2: prostaglandin E2; Erk: extracellular signal-regulated kinases
This review concludes that pharmacologic-grade CS supplements may have clinically significant benefits when properly standardized. However, high-quality evidence from properly designed clinical trials is still needed to draw definitive conclusions about clinical efficacy in OA.”
https://www.cureus.com/articles/162218-chondroitin-sulfate-supplements-for-osteoarthritis-a-critical-review#!/ “Chondroitin Sulfate Supplements for Osteoarthritis: A Critical Review”
A rodent study induced arthritis, then investigated treatment effects of daily glucosamine sulfate (GS, 300 mg), CS (300 mg, a human equivalent dose of 3.4 grams ((300 mg x .162) x 70 kg), and GS+CS = GC combination:
“Rheumatoid arthritis (RA) is an autoimmune disease that affects joints primarily, causing cartilage and bone degeneration as well as functional disability. This study found that both GS and CS could reduce symptoms of RA-related joint inflammation and swelling to some extent, with the effect of GC being more apparent, providing a theoretical foundation for expanding usage of GS and CS.
We discovered that gut bacteria enriched in the RA model were mostly strongly correlative with pro-inflammatory cytokines, right paw volume, and pathological score using correlation analysis. After GS, CS, and GC intervention, these bacteria enriched in the RA model recovered, with GC having the most apparent beneficial impact.
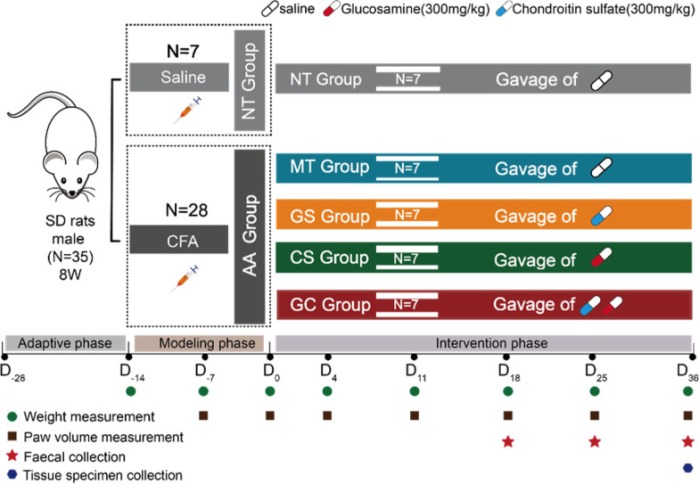
Gut microbiota dysbiosis could be recovered before improvement of joint symptoms after intervention. Our findings also indicated that GC might inhibit LPS-producing bacteria and activation of the TLR-4/NF-κB pathway, alleviating RA-induced joint inflammation and ameliorating joint swelling and injury.”
https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/s12986-023-00735-2 “Combined treatment with glucosamine and chondroitin sulfate improves rheumatoid arthritis in rats by regulating the gut microbiota”
A human equivalent of this study’s treatment duration using the maximum lifespan method is (36 days x 32.2) = 1159 days or 38.6 months.
A rodent study worked backwards from a 70 mg/kg daily human CS dose (70 mg x 70 kg = 4.9 grams):
“A female rat model of osteoporosis was established by feeding a low-calcium diet. Intestinal microbiota abundance, fecal and plasma metabolite expression levels of rats fed a basal diet (N), a low-calcium diet (C), a low-calcium diet plus calcium carbonate (Ca), and a low-calcium diet plus chondroitin sulfate (CS) were compared.
Results showed that compared with the low calcium group, calcium content and bone mineral density of femur were significantly increased in the calcium carbonate and chondroitin sulfate groups. In addition to estrogen, low testosterone bioavailability may also be more likely to lead to fracture. Levels of plasma testosterone and stearic content in normal feeding rats were significantly higher than those in the other groups, indicating that plasma testosterone and stearic content in feces of normal feeding rats were decreased due to long-term low calcium levels, and supplementation of calcium and CS could not be recovered.
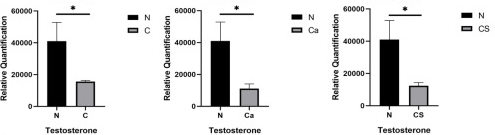
Chronic low-grade inflammation has been identified as the root cause of many diseases, including osteoporosis. We identified bacteria and metabolites behind this change, especially lipid metabolism, and discussed their relevance to bone health.
According to previous studies, as a human therapeutic agent, CS alone has low bioavailability, which is only about 0–13% of total oral intake. This study provides a new strategy to elucidate the molecular mechanism of osteoporosis and to search for potential biomarkers.”
https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/s12986-023-00726-3 “Chondroitin sulfate alleviates osteoporosis caused by calcium deficiency by regulating lipid metabolism”
A human equivalent of this study’s treatment duration is (8 weeks x 32.2) = 257.6 weeks or 5 years.
A rodent study made old female rats out of young female rats by removing their ovaries, then tested different osteoporosis treatment effects:
“In this study, CS oligosaccharides (CSOs) were enzymatically prepared through the lysis of CS by a chondroitinase from Microbacterium sp. strain.
- 12 weeks’ intragastric administration of Caltrate D (250 mg/kg/d), CS or CSOs (500 mg/kg/d, 250 mg/kg/d, 125 mg/kg/d) could regulate disorder of serum indices, recover mechanical strength and mineral content of bone, improve cortical bones’ density and the number and length of trabecular bones in OVX rats.
- Both CS and CSOs in 500 mg/kg/d and 250 mg/kg/d could restore more efficiently serum indices, bone fracture deflection and femur Ca than Caltrate D.
- As compared with CS at the same dosage, CSOs exhibited a more significant alleviating effect. The possible reason might be that the lower molecular weight of CSOs facilitated body absorption and thereafter bioactivity improvement.”
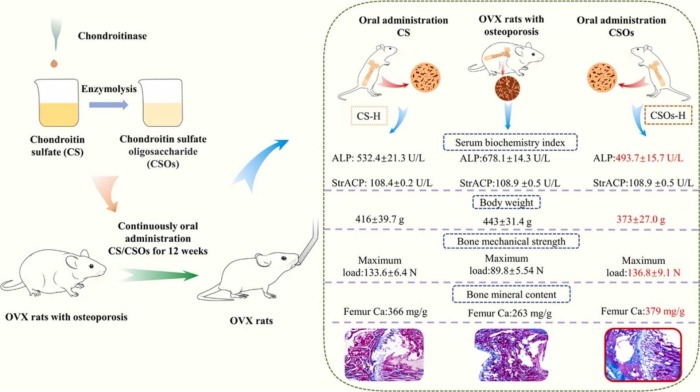
https://www.sciencedirect.com/science/article/pii/S0753332223006844 “Enzymatic preparation of chondroitin sulfate oligosaccharides and its alleviating effect on ovariectomy-induced osteoporosis in rats”
A human equivalent of the above-pictured high CS 500 mg daily dose is (500 mg x .162) x 70 kg = 5.7 grams. A human equivalent of this study’s treatment duration is (12 weeks x 32.2) = 386.4 weeks or 7.4 years.



























 Herding, the story of 2020
Herding, the story of 2020
