
Month: June 2022
Gut microbiota therapy
This June 2022 review cited twenty 2022 papers for relationships between Parkinson’s disease and gut microbiota:
“Clinical diagnosis of PD is based on typical motor symptoms, and novel diagnostic biomarkers have been developed such as imaging markers, and α-synuclein fluid and tissue markers. Multimorbidity of non-motor disorders heighten the risk of adverse outcomes for patients with PD, which usually appear 20 years before onset of motor symptoms.
The gut microbiota is intimately connected to occurrence, development, and progression of PD, especially in early stages. A better understanding of the microbiota–gut–brain axis in PD can provide an opportunity to monitor an individual’s health by manipulating gut microbiota composition.
Several approaches like administration of probiotics, psychobiotics, prebiotics, synbiotics, postbiotics, FMT, and dietary modifications have been tried to mitigate dysbiosis-induced ill effects and alleviate PD progression.

Epidemiological studies have reported that diet affects (positively or negatively) onset of neurodegenerative disorders. Evidence suggests that diet composition’s effects on brain health is not due to diet-induced inflammatory response, but because of its effects on the gut microbiome.
Dysbiotic gut microbiota (including altered microbial metabolites) may play crucial roles in PD via various mechanisms, such as:
- Increased intestinal permeability;
- Aggravated intestinal inflammation and neuroinflammation;
- Abnormal aggregation of α-synuclein fibrils;
- Imbalanced oxidative stress; and
- Decreased neurotransmitters production.
Future studies are essential to further elucidate cause-effect relationships between gut microbiota and PD, improved PD therapeutic and diagnostic options, disease progression tracking, and patient stratification capabilities to deliver personalized treatment and optimize clinical trial designs.”
https://www.frontiersin.org/articles/10.3389/fimmu.2022.937555/full “Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease”

Sending lifespan signals
A second update to Signaling pathways and aging:
“At 40 months old, 3 treated rats are still alive, and all 8 control rats have died. This study started in February of 2021 when all female subjects were 24 months old.
The study will continue until all rats are dead. Animals receive intravenous E5 every 90 days.”
The initial study was all males, and this first follow-on study is all females. Both studies’ treatment groups started at human-equivalent ages of 65-67 years.
Seven weeks ago there were 6 treated rats and 4 control rats alive. Subject deaths since then were at human-equivalent ages of 100-110 years. Rat maximum lifespan is 45 months, so we’ll find out later this year how the treatment affects lifespan.
“A new study of intravenous E5 in rats began last week, with 24 Sprague Dawley rats which are 18 to 20 months old. 12 rats are in the treated group and 12 are in the control group.
Of these, 6 are males and 6 are females in each group. The dosing cycle has been changed to 45 days.”

Taurine week #7: Brain
Finishing a week’s worth of 2022 taurine research with two reviews of taurine’s brain effects:
“We provide a overview of brain taurine homeostasis, and review mechanisms by which taurine can afford neuroprotection in individuals with obesity and diabetes. Alterations to taurine homeostasis can impact a number of biological processes such as osmolarity control, calcium homeostasis, and inhibitory neurotransmission, and have been reported in both metabolic and neurodegenerative disorders.
Models of neurodegenerative disorders show reduced brain taurine concentrations. On the other hand, models of insulin-dependent diabetes, insulin resistance, and diet-induced obesity display taurine accumulation in the hippocampus. Given cytoprotective actions of taurine, such accumulation of taurine might constitute a compensatory mechanism that attempts to prevent neurodegeneration.

Taurine release is mainly mediated by volume-regulated anion channels (VRAC) that are activated by hypo-osmotic conditions and electrical activity. They can be stimulated via glutamate metabotropic (mGluR) and ionotropic receptors (mainly NMDA and AMPA), adenosine A1 receptors (A1R), and metabotropic ATP receptors (P2Y).
Taurine mediates its neuromodulatory effects by binding to GABAA, GABAB, and glycine receptors. While taurine binding to GABAA and GABAB is weaker than to GABA, taurine is a rather potent ligand of the glycine receptor. Reuptake of taurine occurs via taurine transporter TauT.
Cytoprotective actions of taurine contribute to brain health improvements in subjects with obesity and diabetes through various mechanisms that improve neuronal function, such as:
- Modulating inhibitory neurotransmission, which promotes an excitatory–inhibitory balance;
- Stimulating antioxidant systems; and
- Stabilizing mitochondria energy production and Ca2+ homeostasis.”
https://www.mdpi.com/2072-6643/14/6/1292/htm “Taurine Supplementation as a Neuroprotective Strategy upon Brain Dysfunction in Metabolic Syndrome and Diabetes”
A second review focused on taurine’s secondary bile acids produced by gut microbiota:
“Most neurodegenerative disorders are diseases of protein homeostasis, with misfolded aggregates accumulating. The neurodegenerative process is mediated by numerous metabolic pathways, most of which lead to apoptosis. Hydrophilic bile acids, particularly tauroursodeoxycholic acid (TUDCA), have shown important anti-apoptotic and neuroprotective activities, with numerous experimental and clinical evidence suggesting their possible therapeutic use as disease-modifiers in neurodegenerative diseases.
Biliary acids may influence each of the following three mechanisms through which interactions within the brain-gut-microbiota axis take place: neurological, immunological, and neuroendocrine. These microbial metabolites can act as direct neurotransmitters or neuromodulators, serving as key modulators of the brain-gut interactions.
The gut microbial community, through their capacity to produce bile acid metabolites distinct from the liver, can be thought of as an endocrine organ with potential to alter host physiology, perhaps to their own favour. Hydrophilic bile acids, currently regarded as important hormones, exert modulatory effects on gut microbiota composition to produce secondary bile acids which seem to bind a number of receptors with a higher affinity than primary biliary acids, expressed on many different cells.

TUDCA regulates expression of genes involved in cell cycle regulation and apoptotic pathways, promoting neuronal survival. TUDCA:
- Improves protein folding capacity through its chaperoning activity, in turn reducing protein aggregation and deposition;
- Reduces reactive oxygen species production, leading to protection against mitochondrial dysfunction;
- Ameliorates endoplasmic reticulum stress; and
- Inhibits expression of pro-inflammatory cytokines, exerting an anti-neuroinflammatory effect.
Although Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and cerebral ischemia have different disease progressions, they share similar pathways which can be targeted by TUDCA. This makes this bile acid a potentially strong therapeutic option to be tested in human diseases. Clinical evidence collected so far has reported comprehensive data on ALS only.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9166453/ “Tauroursodeoxycholic acid: a potential therapeutic tool in neurodegenerative diseases”
Taurine week #6: Stress
Two 2022 rodent studies of taurine’s associations with long-term stress, starting with a chronic restraint stress model:
“We show that chronic restraint stress can lead to hyperalgesia accompanied by changes in gut microbiota that have significant gender differences. Corresponding changes of bacteria can further induce hyperalgesia and affect different serum metabolism in mice of the corresponding sex.
Different serum metabolites between pseudo-germ-free mice receiving fecal microbiota transplantation from the chronic restraint stress group and those from the control group were mainly involved in bile secretion and steroid hormone biosynthesis for male mice, and in taurine and hypotaurine metabolism and tryptophan metabolism for female mice.
Effects of gut microbiota transplantation on serum metabolomics of female host: Taurine and hypotaurine metabolism, tryptophan metabolism, serotonergic synapse, arachidonic acid metabolism, and choline metabolism in cancer were the five identified pathways in which these different metabolites were enriched.
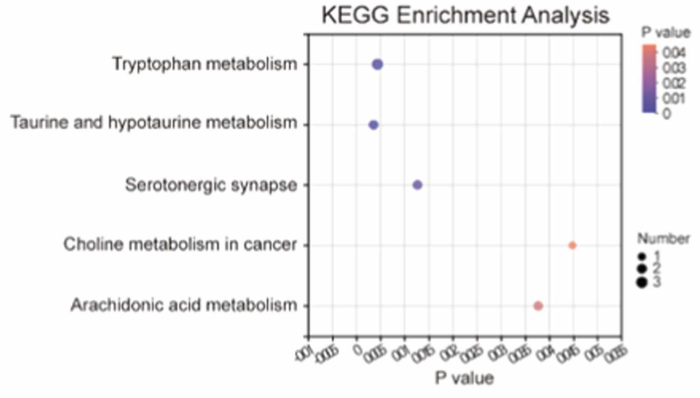
Taurine and hypotaurine play essential roles in anti-inflammation, anti-hypertension, anti-hyperglycemia, and analgesia. Taurine can be used as a diagnostic index for fibromyalgia syndrome and neuropathic pain.
These findings improve our understanding of sexual dimorphism in gut microbiota in stress-induced hyperalgesia and the effect of gut microbiota on blood metabolic traits. Follow-up research will investigate causal relationships between them.”
https://www.sciencedirect.com/science/article/pii/S1043661822000743 “Gut microbiota and its role in stress-induced hyperalgesia: Gender-specific responses linked to different changes in serum metabolites”
Human equivalents:
- A 7-8 month-old mouse would be a 38-42 year-old human.
- A 14-day stress period is about two years for humans.
A second study used a chronic social defeat stress model:
“The level of taurine in extracellular fluid of the cerebral medial prefrontal cortex (mPFC) was significantly reduced in mice with chronic social defeat stress (CSDS)-induced depression. We found that taurine supplementation effectively rescued immobility time during a tail suspension assay and improved social avoidance behaviors in CSDS mice.
Male C57BL/6 J mice (∼ 23 g) and male CD-1 mice aged 7–8 months (∼ 45 g) were used. CD-1 mice were screened for aggressive behavior during social interactions for three consecutive days before the start of the social defeat sessions. Experimental C57BL/6 J mice were subjected to physical interactions with a novel CD-1 mouse for 10 min once per day over 10 consecutive days.
We found significant reductions in taurine and betaine levels in mPFC interstitial fluid of CSDS mice compared with control mice.

We additionally investigated levels of interstitial taurine in chronic restraint stress (CRS) mice, another depressive animal model. After 14 days of CRS treatment, mice showed typical depression-like behaviors, including decreased sucrose preference and increased immobility time. mPFC levels of interstitial taurine were also significantly decreased in CRS mice.
Taurine treatment protected CSDS mice from impairments in dendritic complexity, spine density, and proportions of different types of spines. Expression of N-methyl D-aspartate receptor subunit 2A, an important synaptic receptor, was largely restored in the mPFC of these mice after taurine supplementation.
These results demonstrated that taurine exerted an antidepressive effect by protecting cortical neurons from dendritic spine loss and synaptic protein deficits.”
https://link.springer.com/article/10.1007/s10571-022-01218-3 “Taurine Alleviates Chronic Social Defeat Stress-Induced Depression by Protecting Cortical Neurons from Dendritic Spine Loss”
Human equivalents:
- A 7-8 month-old mouse would be a 38-42 year-old human.
- A 500 mg/kg taurine dose injected intraperitoneally is (.081 x 500 mg) x 70KG = 2.835 g.
- A 10-day stress period is about a year and a half for humans.
Don’t think aggressive humans would have to be twice as large to stress those around them. There may be choices other than enduring a year and a half of that.
Taurine week #5: Blood
Two 2022 papers investigated taurine’s effects in blood, starting with a review of platelets:
“Taurine is the most abundant free amino acid in the human body, with a six times higher concentration in platelets than any other amino acid. It is highly beneficial for the organism, has many therapeutic actions, and is currently approved for heart failure treatment in Japan. Only the lack of large-scale phase 3 clinical trials restricts taurine use as a therapeutic agent in several other pathologies for treatment of which it has been shown to be effective (hypertension, atherosclerosis, stroke, neurodegenerative diseases, metabolic diseases, e.g., diabetes mellitus, and others).
Because taurine was seen as a non-patentable nutrient, the pharmaceutical industry has not shown much interest in its research. Considering that taurine and its analogues display permissible side effects, along with the need of finding new, alternative antithrombotic drugs with minimal side effects and long-term action, the potential clinical relevance of this fascinating nutrient and its derivatives requires further consideration.”
https://www.mdpi.com/2077-0383/11/3/666/htm “Taurine and Its Derivatives: Analysis of the Inhibitory Effect on Platelet Function and Their Antithrombotic Potential”
Figure 1 provided details of taurine and its derivatives’ effects on various processes involved in platelet activation and aggregation.
A second paper was a rodent study:
“To evaluate chronic effects of taurine on cholesterol levels, we analyzed mice fed a taurine-rich diet for 14–16 weeks. Long-term feeding of taurine lowered plasma cholesterol and bile acids without significantly changing other metabolic parameters, but hardly affected these levels in the liver.

Taurine upregulates transcriptional activity of Cyp7a1 by suppressing FGF21 production in the liver. Bile acids are converted from blood cholesterol by CYP7A1, and more efficiently enter enterohepatic circulation via taurine conjugation.
This study shows that long-term feeding of taurine lowers both plasma cholesterol and bile acids, reinforcing that taurine effectively prevents hypercholesterolemia.”
https://www.mdpi.com/1422-0067/23/3/1793/htm “Long-Term Dietary Taurine Lowers Plasma Levels of Cholesterol and Bile Acids”
A human equivalent of this male C57BL/6J mouse 16-week taurine intervention is roughly 17 years. That strain’s male maximum lifespan is around 800 days, and human maximum lifespan is currently 122.5 years.

Taurine week #4: Muscles
Two 2022 papers investigated taurine and skeletal muscles, starting with a rodent study of endurance exercise:
“This study examined effects of taurine on dynamics of blood glucose concentration (BGC) during endurance exercise in rats.
- Blood was collected every 10 min from the jugular vein via cannulation.
- Exercise period was divided by every 40 min into four phases.
- Individual exhaustion time is indicated by arrows (white: CON group [n = 12], black: TAU group [n = 10]) under the X-axis.
- 120-min point is the approximate median where there were significant differences in BGC between groups at exercise point every 10 min (80–150 min).
- †P < 0.05 and #P < 0.05 show significant difference to respective starting points (0 min) in TAU and CON groups.
- Arrow with two heads (↔) shows a significant difference at P < 0.05 between groups at each point.

Observation of BGC confirmed that taurine supplementation delayed the decline in once-elevated BGC during endurance exercise.
Significantly higher levels of free fatty acid in plasma as well as acetyl-carnitine and N-acetyltaurine in skeletal muscle at the 120-min point suggested that taurine supplementation shifted the priority of energy substrate utilization in skeletal muscles to fatty acid oxidation during endurance exercise. Consequent sparing effect of taurine on BGC might contribute to enhancing exercise performance.”
https://link.springer.com/article/10.1007/s00726-021-03110-8 “Taurine supplementation enhances endurance capacity by delaying blood glucose decline during prolonged exercise in rats”
A second rodent study focused on injured muscle:
“We evaluated whether taurine administration in old mice counteracts physiopathological effects of aging in skeletal muscle.
Type I slow-twitch oxidative fibers (expressing the slow isoform of the myosin heavy chain, slow MHC) are more resistant to damage and a variety of atrophic conditions than type IIb fast-twitch glycolytic fibers. In several muscle pathologies, including sarcopenia, the fastest muscle phenotype is more severely compromised when compared with slow-twitch muscles.
- Tibialis anterior (TA) muscles of old mice expressed very low levels of slow-MHC isoform compared to young muscles. Slow-MHC expression increased in muscles of taurine-treated mice.
- Analysis of the fast-MHC isoform revealed that, in the presence of taurine, its expression was significantly upregulated compared to what was observed in TA muscles of old mice that did not receive taurine.
These results suggest that the positive effect of taurine on skeletal muscle homeostasis of aged mice may be mediated by stimulation of the PGC1-α/MEF2C pathway, favoring a possible metabolic shift of myofibers towards the oxidative phenotype, and preserving more susceptible glycolytic fibers.
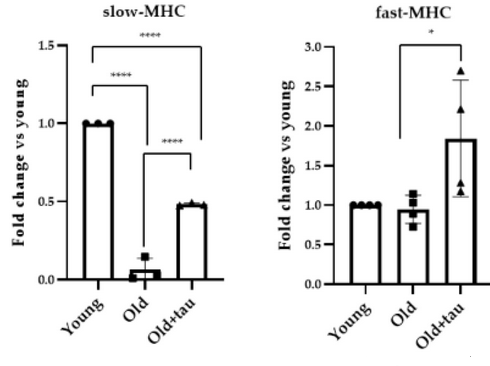
In injured muscle, taurine enhances the regenerative process by downregulating inflammatory response and preserving muscle fiber integrity. Taurine attenuates ROS production in aged muscles by maintaining a proper cellular redox balance, acting as an antioxidant molecule.
These data demonstrate that taurine administration ameliorates the microenvironment, allowing an efficient regenerative process, and attenuation of catabolic pathways related to onset of sarcopenia.”
https://www.mdpi.com/2076-3921/11/5/1016/htm “Taurine Administration Counteracts Aging-Associated Impingement of Skeletal Muscle Regeneration by Reducing Inflammation and Oxidative Stress”
A human equivalent to each daily mouse taurine dose administered over five weeks was (.081 x 100 mg) x 70 kg = .567 g.

Taurine week #3: Organs
Three 2022 papers investigated taurine’s effects on organs, starting with a rodent study of sepsis:
“Sepsis usually causes multiple organ dysfunctions and high mortality. Pathogenesis of sepsis is thought to be driven by hyperactive inflammation following pathogen invasion. If the immune system fails to eradicate pathogens, immune homeostasis is disturbed, leading to an overwhelming inflammation accompanied by immunosuppression.
Metabolomic analysis showed large amounts of taurine in neutrophils and monocytes and a dramatic decrease in taurine levels after lipopolysaccharides (LPS) exposure:

Cecal ligation and puncture (CLP) model mice and CLP plus taurine mice were injected intraperitoneally with saline (200 μl) or taurine (200 mg/kg, in 200 μl) respectively at 6, 24, and 48 h after the operation. Taurine protected septic mice from death, improving tissue injuries in the lung, liver, and kidney by reducing neutrophil infiltration and TNF-α production.

Our data indicate that a supplement with taurine might be a promising therapeutic strategy for sepsis to reduce hyperactive inflammation and improve multiple organ dysfunctions.”
https://www.sciencedirect.com/science/article/abs/pii/S0008874922000272 “Mechanism of taurine reducing inflammation and organ injury in sepsis mice” (not freely available) Thanks to Dr. Liuluan Zhu for providing a copy.
Taurine demonstrated the only decrease in 17 amino acids measured in monocytes above. It was the same story for those amino acids and neutrophils.
A human equivalent to each of three mouse taurine doses administered over two days was (.081 x 200 mg) x 70 kg = 1.134 g. A second dose given at the 12-hour point may have improved treated subjects’ survival, as half of them died before the study’s 24-hour point of a second dose.
A second rodent study was on liver injury:
“We investigated the beneficial effects of taurine on fatty liver injury in vivo induced by tunicamycin, a chemical endoplasmic reticulum (ER) stressor.
The unfolded protein response (UPR) is a protein homeostasis-maintaining system that monitors ER conditions by sensing inadequacy in ER protein folding capacity. The ER is both a protein homeostasis-maintaining system and the primary site of lipid metabolism. The UPR plays vital roles in maintaining metabolic and lipid homeostasis.
Glutathione (GSH), a final byproduct of sulfur-containing amino acid metabolism, is not only a powerful antioxidant, but also a principal redox buffer in the ER. Depletion of reduced glutathione can cause additional oxidative stress.
Cysteine, the metabolic precursor of GSH, is also an essential substrate for taurine synthesis. Utilization of cysteine to generate GSH and taurine is competitive.
In this study, availability of cysteine is favored for GSH synthesis due to sufficient taurine supply. Taurine supplementation restored GSH levels, which were attenuated by tunicamycin treatment, by increasing expression of GCLC, an enzyme mediating GSH synthesis.

The protective effect of taurine on tunicamycin-induced hepatic injury results from its concurrent mitigation of both ER and oxidative stress.”
https://www.mdpi.com/2075-1729/12/3/354/htm “Taurine Ameliorates Tunicamycin-Induced Liver Injury by Disrupting the Vicious Cycle between Oxidative Stress and Endoplasmic Reticulum Stress”
This study provided further evidence for an idea in Treating psychopathological symptoms will somehow resolve causes? that:
“Such positive effects of taurine on glutathione levels may be explained by the fact that cysteine is the essential precursor to both metabolites, whereby taurine supplementation may drive metabolism of cysteine towards GSH synthesis.”
A third rodent study investigated lung pneumonia:
“We evaluated anti-inflammatory effects of taurine derivative N-chlorotaurine (also known as taurine chloramine; TauCl) against LPS-induced pneumonia in obese mice maintained on a high fat diet.
Taurine is present in immune system cells such as macrophages and neutrophils. When an organism is infected by pathogens, immune cells produce hypochlorous acid to kill pathogens. Taurine reacts with excessive hypochlorous acid to produce TauCl, which reduces high levels of hypochlorous acid and its toxicity to surrounding host cells.
Intraperitoneal TauCl suppressed excessive immune response in lungs. TauCl treatment attenuates acute pneumonia-related pulmonary and systemic inflammation, including muscle wasting.”
https://www.mdpi.com/2218-1989/12/4/349/htm “N-Chlorotaurine Reduces the Lung and Systemic Inflammation in LPS-Induced Pneumonia in High Fat Diet-Induced Obese Mice”

Taurine week #2: Bile acids
Two papers investigated taurine’s integration into bile acids, starting with a review:
“Bile acids (BAs) are produced from cholesterol in the liver and are termed primary BAs. Primary BAs are conjugated with glycine and taurine in the liver, and stored in the gallbladder. BAs are released from the gallbladder into the small intestine via food intake to facilitate digestion and absorption of lipids and lipophilic vitamins by forming micelles in the small intestine.
After deconjugation by the gut microbiome, primary BAs are converted into secondary BAs. Most BAs in the intestine are reabsorbed and transported to the liver, where both primary and secondary BAs are conjugated with glycine or taurine and rereleased into the intestine.

Some BAs reabsorbed from the intestine spill into systemic circulation, where they bind to a variety of nuclear and cell-surface receptors in tissues. Some BAs are not reabsorbed and bind to receptors in the terminal ileum.
BAs can affect cell-surface and intracellular membranes, including those of mitochondria and the endoplasmic reticulum. BAs are also hormones or signaling molecules, and can regulate BA, glucose, and lipid metabolism in various tissues, including the liver, pancreas, and both brown and white adipose tissue. BAs also affect the immune system.
BAs can affect the nervous system. More than 20 BAs have been detected in the brain of humans and rodents. The brain communicates with the gut and gut microbiome through BAs.”
https://www.mdpi.com/2076-2607/10/1/68/htm “Physiological Role of Bile Acids Modified by the Gut Microbiome”
Reference 56 was a human study:
“Centenarians (individuals aged 100 years and older) have a decreased susceptibility to ageing-associated illnesses, chronic inflammation, and infectious diseases. Centenarians have a distinct gut microbiome enriched in microorganisms that are capable of generating unique secondary bile acids.
We identified centenarian-specific gut microbiota signatures and defined bacterial species as well as genes and/or pathways that promote generation of isoLCA, 3-oxoLCA, 3-oxoalloLCA, and isoalloLCA. To our knowledge, isoalloLCA is one of the most potent antimicrobial agents that is selective against Gram-positive microorganisms, including multidrug-resistant pathogens, suggesting that it may contribute to maintenance of intestinal homeostasis by enhancing colonization-resistance mechanisms.”
https://www.nature.com/articles/s41586-021-03832-5 “Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians” (not freely available)
A few more papers will be coming on taurine and bile acids. I haven’t seen one investigate both taurine and glycine treatments to aid bile acid in achieving therapeutic results.

Taurine week #1: Biomarkers
It’s been a while since I curated taurine research. Read at least a week’s worth of 2022 papers last weekend.
Let’s start with two studies that didn’t supplement with taurine, but found it was a biomarker. The first was a rodent study that treated a high fat diet with blood pressure medicine:
“Non-alcoholic fatty liver disease (NAFLD) is a main form of chronic liver disease, and has been the leading cause of liver transplantation. Epidemiological evidence uncovered bidirectional and causal relationships between NAFLD and hypertension.
Evidence suggests that gut dysbiosis can be a driving force for NAFLD and hypertension, despite pathogenesis of NAFLD and hypertension fundamental differences, as they often present similar aberrant microbiota. We found that amlodipine besylate and amlodipine aspartate:
- Exerted their hepatoprotective activities through modulating fatty acid metabolism without influencing oxidative and endoplasmic reticulum stress;
- Decreased serum transaminases, hepatic fat deposits, and liver inflammation, and showed improvements in plasma lipid profiles; and
- Gut microbiota had higher abundance of functional genes involved in taurine and hypotaurine metabolism.

Overall, these results led us to propose that targeting gut microbiota and the taurine and hypotaurine metabolism pathway may be a feasible preventive strategy for patients with NAFLD and hypertension.”
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15768 “Amlodipine, an anti-hypertensive drug, alleviates non-alcoholic fatty liver disease by modulating gut microbiota”
A second rodent study investigated garlic compounds’ effects on a high fat diet:
“Garlic organosulfur compounds (OSCs) have been shown to be major components responsible for garlic’s health benefits. However, the composition and function of garlic OSCs are damaged due to various processing and cooking methods during food preparation.
In this study garlic alliinase was deactivated to obtain stable garlic OSCs. We made two preparations of alliinase-free garlic powders based on their OSCs and fructan contents. OSCs concentrations of alliinase-free garlic powder 1 (G1) and alliinase-free garlic powder 2 (G2) differ by approximately 2-fold, 20.889 mg/g and 43.869 mg/g, respectively.

Mice fed with lipid and glucose metabolic disorder-inducing Western diet (WD) revealed that stable garlic OSCs prevented the disorder by increasing relative abundance of gut Bacteroides acidifaciens. Both G1 and G2 significantly increased fecal levels of taurine, with G2 being significantly better than G1.
Garlic OSCs inhibited dyslipidemia and fatty liver by increasing taurine and subsequently promoting hepatic fatty acid β-oxidation. Results of this study demonstrate that the preventive effect of garlic OSCs on WD-induced metabolic disorder is attributed to enhanced growth of Bacteroides acidifaciens and consequent increase in taurine.”
https://pubs.acs.org/doi/10.1021/acs.jafc.2c00555 “Natural Garlic Organosulfur Compounds Prevent Metabolic Disorder of Lipid and Glucose by Increasing Gut Commensal Bacteroides acidifaciens” (not freely available) Thanks to Dr. Hisham R. Ibrahim for providing a copy.

Betaine and diabetes
Two 2022 papers on betaine’s effects, starting with a review:
“Rodent studies provide evidence that betaine effectively limits many diabetes-related disturbances.
- Betaine therapy improves glucose tolerance and insulin action, which is strongly associated with changes in insulin-sensitive tissues, such as skeletal muscle, adipose tissue, and liver.
- Betaine supplementation positively affects multiple genes, which expression is dysregulated in diabetes.
- AMP-activated protein kinase is thought to play a central role in the mechanism underlying anti-diabetic betaine action.
- Studies with animal models of type 2 diabetes have shown that betaine exerts anti-inflammatory and anti-oxidant effects, and also alleviates endoplasmic reticulum stress.

These changes contribute to improved insulin sensitivity and better blood glucose clearance. Results of animal studies encourage exploration of therapeutic betaine efficacy in humans with type 2 diabetes.”
https://www.sciencedirect.com/science/article/pii/S0753332222003353 “The anti-diabetic potential of betaine. Mechanisms of action in rodent models of type 2 diabetes”
Reference 31 was a human study:
“Few studies on humans have comprehensively evaluated intake composition of methyl-donor nutrients choline, betaine, and folate in relation to visceral obesity (VOB)-related hepatic steatosis (HS), the hallmark of non-alcoholic fatty liver diseases.
- Total choline intake was the most significant dietary determinant of HS in patients with VOB.
- Combined high intake of choline and betaine, but not folate, was associated with an 81% reduction in VOB-related HS.
- High betaine supplementation could substitute for choline and folate to normalize homocysteine levels under methyl donor methionine-restriction conditions.
- Preformed betaine intake from whole-grain foods and vegetables can lower obesity-increased choline and folate requirements by sparing choline oxidation for betaine synthesis and folate for methyl donor conversion in one-carbon metabolism.
Our data suggest that combined dietary intake of choline and betaine reduces the VOB-related HS risk in a threshold-dependent manner.”
https://www.mdpi.com/2072-6643/14/2/261/htm “Optimal Dietary Intake Composition of Choline and Betaine Is Associated with Minimized Visceral Obesity-Related Hepatic Steatosis in a Case-Control Study”
Increasing betaine intake to lower choline and folate requirements was similar to an idea in Treating psychopathological symptoms will somehow resolve causes? that:
“Such positive effects of taurine on glutathione levels may be explained by the fact that cysteine is the essential precursor to both metabolites, whereby taurine supplementation may drive metabolism of cysteine towards GSH synthesis.”
I came across this first paper by it citing All about the betaine, Part 2:
“This review focuses on biological and beneficial effects of dietary betaine (trimethylglycine), a naturally occurring and crucial methyl donor that restores methionine homeostasis in cells. Betaine is endogenously synthesized through metabolism of choline, or exogenously consumed through dietary intake.
Human intervention studies showed no adverse effects with 4 g/day supplemental administration of betaine in healthy subjects. However, overweight subjects with metabolic syndrome showed a significant increase in total and LDL-cholesterol concentrations. These effects were not observed with 3 g/day of betaine administration.
Betaine exerts significant therapeutic and biological effects that are potentially beneficial for alleviating a diverse number of human diseases and conditions.”
https://www.mdpi.com/2079-7737/10/6/456/htm “Beneficial Effects of Betaine: A Comprehensive Review”

The oligosaccharide stachyose
Two 2022 stachyose papers to follow on to Don’t take Beano if you’re stressed, which studied raffinose. Stachyose is in the raffinose oligosaccharide group with similar characteristics, and its content is usually larger in legumes. First is a rodent study:
“Stress can activate the hypothalamic–pituitary–adrenal (HPA) axis and elevate glucocorticoids in the body (cortisol in humans and corticosterone in rodents). Glucocorticoid receptors are abundant in the hippocampus, and play an important role in stress-induced cognition alteration.
Corticosterone is often used to model cognitive impairment induced by stress. Long-term potentiation (LTP) deficit and cognitive impairment always coexist in stress models, and LTP impairment is often considered as one mechanism for stress-induced cognitive deficits.
N-methyl-D-aspartate (NMDA) receptors play critical roles both in normal synaptic functions and excitotoxicity in the central nervous system. D-serine, a coactivator of NMDA receptors, plays an important role in brain function.
In this study, we focused on effects of stachyose, on LTP impairment by corticosterone, gut flora, and the D-serine pathway.

Data in this study showed that 7-consecutive-day intragastric (i.g.) administration of stachyose had protective effect. There was little effect via intracerebroventricular (i.c.v.) and intraperitoneal (i.p.) administration.
To disturb gut flora, a combination of non-absorbable antibiotics (ATB) were applied. Results showed that ATB canceled the protective effect of stachyose without affecting LTP in control and corticosterone-treated mice, suggesting that stachyose may display its protective effects against LTP impairment by corticosterone via gut flora.
Further study is needed to uncover the relation between gut flora and the D-serine metabolic pathway.”
https://www.frontiersin.org/articles/10.3389/fphar.2022.799244/full “Stachyose Alleviates Corticosterone-Induced Long-Term Potentiation Impairment via the Gut–Brain Axis”
One of this study’s references was Eat oats and regain cognitive normalcy.
A stachyose clinical trial is expected to complete this month:
“In the stachyose intervention group, each person took 5 g of stachyose daily before breakfast. Administration method was 100 ml of drinking water dissolved and taken orally for two months. Each person in the placebo control group took the same amount of maltodextrin daily. Stool samples of the 36 subjects were collected weekly.
Primary outcome measures:
- Expression of microRNA; and
- Structure of gut microbiota.”
https://clinicaltrials.gov/ct2/show/NCT05392348 “Regulatory Effect of Stachyose on Gut Microbiota and microRNA Expression in Human”

The misnomer of nonessential amino acids
Three papers, starting with a 2022 review:
“Ideal diets must provide all physiologically and nutritionally essential amino acids (AAs).
Proposed optimal ratios and amounts of true digestible AAs in diets during different phases of growth and production. Because dynamic requirements of animals for dietary AAs are influenced by a plethora of factors, data below as well as the literature serve only as references to guide feeding practices and nutritional research.

Nutritionists should move beyond the ‘ideal protein’ concept to consider optimum ratios and amounts of all proteinogenic AAs in diets for mammals, birds, and aquatic animals, and, in the case of carnivores, also taurine. This will help formulate effectively low-protein diets for livestock (including swine and high-producing dairy cattle), poultry, fish, and crustaceans, as well as zoo and companion animals.”
https://journals.sagepub.com/doi/10.1177/15353702221082658 “The ‘ideal protein’ concept is not ideal in animal nutrition”
A second 2022 review focused on serine:
“The main dietary source of L-serine is protein, in which L-serine content ranges between 2 and 5%. At the daily intake of ~1 g protein per kg of body weight, the amount of serine obtained from food ranges between 1.4 and 3.5 g (13.2–33.0 mmol) per day in an adult.
Mechanisms of potential benefits of supplementing L-serine include increased synthesis of sphingolipids, decreased synthesis of 1-deoxysphingolipids, decrease in homocysteine levels, and increased synthesis of cysteine and its metabolites, including glutathione. L-serine supplementation has been suggested as a rational therapeutic approach in several disorders, particularly primary disorders of L-serine synthesis, neurodegenerative disorders, and diabetic neuropathy.
Unfortunately, the number of clinical studies evaluating dietary supplementation of L-serine as a possible therapy is small. Studies examining therapeutic effects of L-serine in CNS injury and chronic renal diseases, in which it is supposed that L-serine weakens glutamate neurotoxicity and lowers homocysteine levels, respectively, are missing.”
https://www.mdpi.com/2072-6643/14/9/1987/htm “Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid”
A 2021 review subject was D-serine, L-serine’s D-isoform:
“The N-methyl-D-aspartate glutamate receptor (NMDAR) and its co-agonist D-serine are currently of great interest as potential important contributors to cognitive function in normal aging and dementia. D-serine is necessary for activation of NMDAR and in maintenance of long-term potentiation, and is involved in brain development, neuronal connectivity, synaptic plasticity, and regulation of learning and memory.
The source of D-amino acids in mammals was historically attributed to diet or intestinal bacteria until racemization of L-serine by serine racemase was identified as the endogenous source of D-serine. The enzyme responsible for catabolism (breakdown) of D-serine is D-amino acid oxidase; this enzyme is most abundant in cerebellum and brainstem, areas with low levels of D-serine.
Activation of the NMDAR co-agonist-binding site by D-serine and glycine is mandatory for induction of synaptic plasticity. D-serine acts primarily at synaptic NMDARs whereas glycine acts primarily at extrasynaptic NMDARs.
In normal aging there is decreased expression of serine racemase and decreased levels of D-serine and down-regulation of NMDARs, resulting in impaired synaptic plasticity and deficits in learning and memory. In contrast, in AD there appears to be activation of serine racemase, increased levels of D-serine and overstimulation of NMDARs, resulting in cytotoxicity, synaptic deficits, and dementia.”
https://www.frontiersin.org/articles/10.3389/fpsyt.2021.754032/full “An Overview of the Involvement of D-Serine in Cognitive Impairment in Normal Aging and Dementia”

Fueling a gut fire
This 2022 article commented on a human / rodent study of gut dysbiosis:
“Crohn’s disease (CD) is a chronic disease that causes inflammation in the gastrointestinal track. Together with ulcerative colitis, another major type of inflammatory bowel disease (IBD), these intestinal disorders affect millions of people in the U.S. and worldwide.
Excessive T helper 1 (Th1) and Th17 cell responses have been documented to act as important mediators of CD pathogenesis. An imbalance between regulatory T (Treg) cells and effector T cells in the intestinal tissue microenvironment is crucial to promote gut inflammation in CD.
Lysophosphatidylserine (LysoPS) exaggerates intestinal inflammation by fueling IFNγ-producing Th1 cells via metabolic reprogramming and chromatin modification (panel A). While this work has provided novel functional insights into dysbiotic microbiota–derived LysoPS in CD pathogenesis (panel B), it also raises several questions.

By employing multiple animal colitis models, the authors have shown that administration of LysoPS was detrimental in T cell–driven colitis, while having no significant impact on pathogenesis of T cell–independent dextran sodium sulfate–induced colitis.
Considering the complex nature of LysoPS in regulating responses of different immune cell types in a given tissue environment under a particular physiological or pathological condition, more research is needed to elucidate the precise role of LysoPS in CD before targeting these multifunctional bioactive lipids to treat human gastrointestinal disorders becomes a reality.”
https://doi.org/10.1084/jem.20220723 “Fueling the fire in the gut”
The referenced study:
- “We identified key metabolites derived from dysbiotic microbiota that induce enhanced Th1 responses and exaggerate colitis in mouse models.
- Patients with CD showed elevated LysoPS concentration in their feces, accompanied by a higher relative abundance of microbiota possessing a gene encoding the phospholipid-hydrolyzing enzyme phospholipase A.
- Our findings elaborate on the mechanism by which metabolites elevated in patients with CD harboring dysbiotic microbiota promote Th1-mediated intestinal pathology.”
https://doi.org/10.1084/jem.20211291 “Lysophosphatidylserines derived from microbiota in Crohn’s disease elicit pathological Th1 response”
When standard DSS and TNBS models of colitis don’t account for observed effects, other avenues need to be investigated. Relationships with our gut microbiota are complicated.
