This 2019 Stanford human study developed an aging clock using blood plasma proteins:
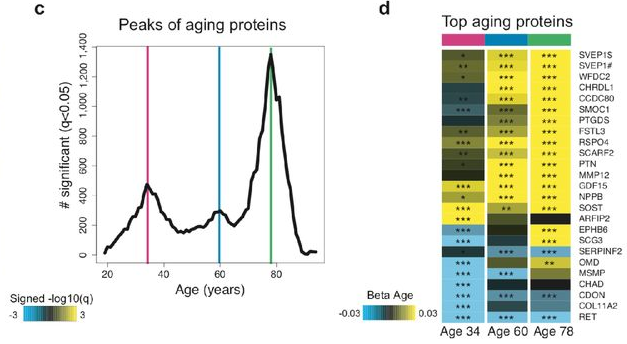
“We measured 2,925 plasma proteins from 4,331 young adults to nonagenarians [18 – 95] and developed a novel bioinformatics approach which uncovered profound non-linear alterations in the human plasma proteome with age. Waves of changes in the proteome in the fourth, seventh, and eighth decades of life reflected distinct biological pathways, and revealed differential associations with the genome and proteome of age-related diseases and phenotypic traits.
To determine whether the plasma proteome can predict chronological age and serve as a “proteomic clock,” we used 2,858 randomly selected subjects to fine-tune a predictive model that was tested on the remaining 1,473 subjects. We identified a sex-independent plasma proteomic clock consisting of 373 proteins. Subjects that were predicted younger than their chronologic age based on their plasma proteome performed better on cognitive and physical tests.
The 3 age-related crests were comprised of different proteins. Few proteins, such as GDF15, were among the top 10 differentially expressed proteins in each crest, consistent with its strong increase across lifespan. Other proteins, like chordin-like protein 1 (CHRDL1) or pleiotrophin (PTN), were significantly changed only at the last two crests, reflecting their exponential increase with age.
We observed a prominent shift in multiple biological pathways with aging:
- At young age (34 years), we observed a downregulation of proteins involved in structural pathways such as the extracellular matrix. These changes were reversed in middle and old ages (60 and 78 years, respectively).
- At age 60, we found a predominant role of hormonal activity, binding functions and blood pathways.
- At age 78, key processes still included blood pathways but also bone morphogenetic protein signaling, which is involved in numerous cellular functions, including inflammation.
These results suggest that aging is a dynamic, non-linear process characterized by waves of changes in plasma proteins that are reflective of a complex shift in the activity of biological processes.”
https://www.biorxiv.org/content/10.1101/751115v1.full “Undulating changes in human plasma proteome across lifespan are linked to disease”
A non-critical review of the study was published by the Life Extension Advocacy Foundation. Frequent qualifiers like “could,” “may,” and “possible” were consistent with the confirmation biases of their advocacy.
There were several misstatements of what the study did, including the innumerate:
- “used around half of the participant data to build a “proteomic clock”
- tested it on the other half of the participants
- a total of 3000 proteins”
Per the above study quotation, the numbers were actually:
- Closer to two thirds (2,858 ÷ 4,331), not “around half”;
- The other third (1,473 ÷ 4,331), not “the other half”; and
- 2,925 not 3000.
The final paragraph and other parts of the review bordered on woo. Did a review of the findings have to fit LEAF’s perspective?
In contrast, Josh Mitteldorf did his usual excellent job of providing contexts for the study with New Aging Clock based on Proteins in the Blood, emphasizing comparisons with epigenetic clock methodologies:
“For some of the proteins that feature prominently in the clock, we have a good understanding of their metabolic function, and for the most part they vindicate my belief that epigenetic changes are predominantly drivers of senescence rather than protective responses to damage.
Wyss-Coray compared the proteins in the new (human) proteome clock with the proteins that were altered in the (mouse) parabiosis experiments, and found a large overlap [46 proteins change in the same direction and define a conserved aging signature]. This may be the best evidence we have that the proteome changes are predominantly causal factors of senescence.

Almost all the proteins identified as changing rapidly at age 78 are increasing. In contrast, a few of the fastest-changing proteins at age 60 are decreasing (though most are increasing). GDF15 deserves a story of its own.
The implication is that a more accurate clock can be constructed if it incorporates different information at different life stages. None of the Horvath clocks have been derived based on different CpG sites at different ages, and this suggests an opportunity for a potential improvement in accuracy.”
A commentator linked the below study:
https://www.sciencedirect.com/science/article/pii/S0092867419308323 “GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance” (not freely available)
which prompted his response:
“Thanks, Lee! This is just the kind of specific information that I was asking for. It would seem we should construct our clocks without GDF15, which otherwise might loom large.”
[…] 연구 결과에 따르면 급격한 노화가 34, 60, 78세에 한 번씩 온다고 하는데요. 이 연구 […]
Hi! Thanks for commenting.
Yes, people have to get ready for it, even though we won’t be completely ready. For example, I played the best round of golf I’ve ever played in a competitive tournament two weeks after I turned 60. Two years later, I couldn’t play golf anymore.