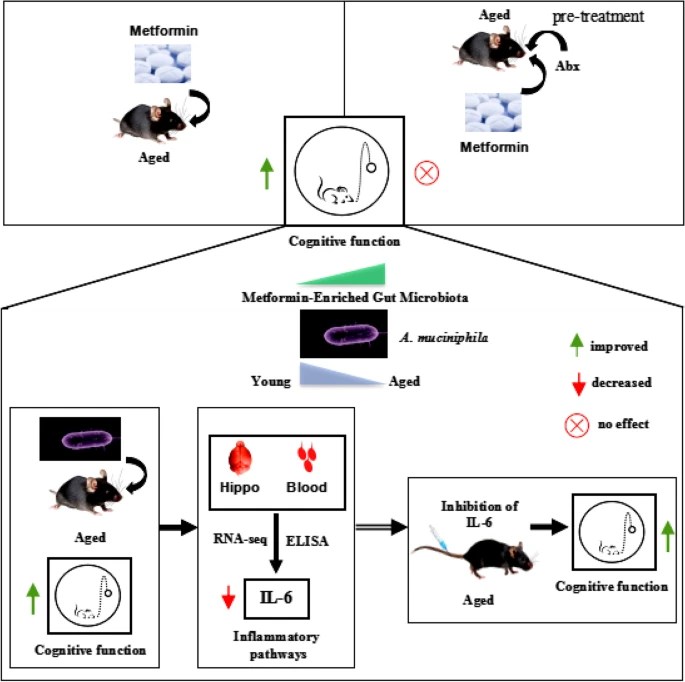
This 2023 study investigated a dozen fruits and vegetables processed with three methods and freezer times for impacts on their sixteen main nutrients. I’ll focus on sulforaphane:
“This paper compares how different processing methods (pasteurization vs. high hydrostatic pressure processing or pascalization) affect phytochemical concentrations of a complex mixture of fruits and vegetables, and investigates how these methods influence their stability during freezing and over time in frozen storage. Phytochemicals tested were vitamin C, quercetin-3-glucoside, delphinidin-3-glucoside, cyanidin-3-glucoside, peonidin-3-glucoside, catechin, epigallocatechin-3-gallate, epicatechin, epicatechin gallate, chlorogenic acid, sulforaphane, resveratrol, lycopene, lutein, alpha-carotene, and beta-carotene.
After freezing to −18 °C, one bottle from each condition was immediately removed from the freezer and thawed at 4 °C, which took about two days. Measurements at t = 0 for the fresh and frozen condition were technically made two days after processing.

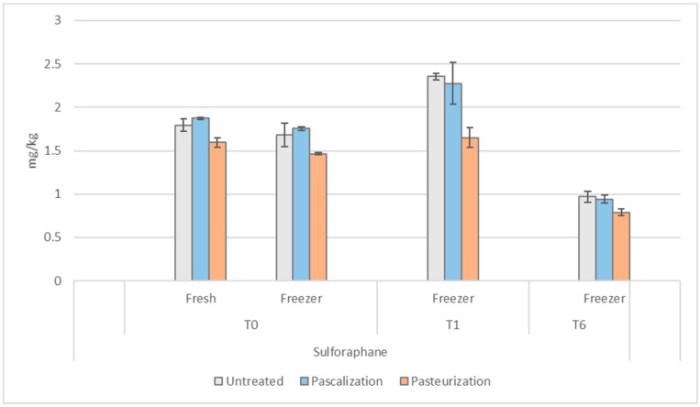
The effect of immediate freezing and thawing on broccoli, cauliflower, and Brussels sprouts sulforaphane levels was consistent despite the processing method (−6% for pascalized and untreated samples, and −8% for pasteurized) at t = 0. Pasteurized samples at t = 0 were 11% lower in sulforaphane than untreated in fresh samples and 13% lower in frozen.
At one month in the freezer, levels of sulforaphane increased in each processing method from t = 0:
- Untreated by +18%;
- Pascalized by +57%; and
- Pasteurized by +94%.
At six months in the freezer, sulforaphane levels in all samples decreased below their t = 0 levels:
- Untreated by -31%;
- Pascalized by -35%; and
- Pasteurized by -35%.
Optimal processing method seems to vary based on the phytochemical of interest. These impacts should be considered to produce foods aimed at preventing chronic disease development.'”
https://www.mdpi.com/2076-3921/12/6/1252 “Impact of Processing Method and Storage Time on Phytochemical Concentrations in an Antioxidant-Rich Food Mixture”
Untreated samples’ sulforaphane took a hit in this study from fresh levels to initial freezing at -18°C then thawing for two days at 4°C. Untreated levels recovered after a month to be more than their two-day levels, but lowered again after six months.
My refrigerator / freezer has one control for both compartments. Pretty sure the freezer can’t get to 0°F / -18°C without ruining refrigerator fruits and vegetables.
In any event, a (1 – .06) x 1.18 = +11% sulforaphane gain after a month isn’t worth my effort. We can increase sulforaphane more than 1100% by microwaving broccoli sprouts in a 1000W microwave on full power for 35 seconds to 60°C (140°F) per Week 6 of Changing an inflammatory phenotype with broccoli sprouts.