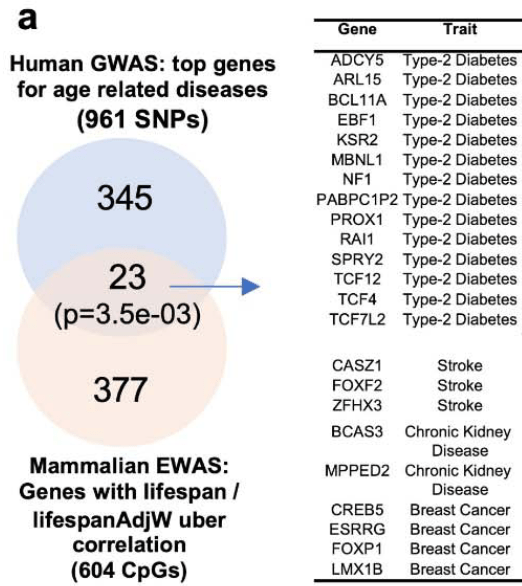
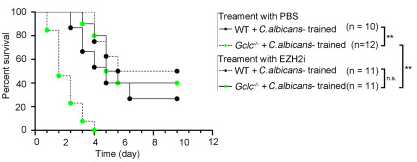
A 2022 McGill University rodent study couldn’t replicate Part 1 findings:
“We find that using similar mouse models of trained immunity induced by:
- Live vaccination (BCG);
- PAMPs (β-glucan); or
- Infection (C. albicans),
protection against:
- Viral (influenza virus);
- Bacterial (Mycobacterium tuberculosis (Mtb)); or
- Fungal (C. albicans)
infections was the same between offspring of trained and non-trained parents.

BCG vaccination in the offspring of vaccinated parents does not enhance trained immunity in macrophages.
a) Mice were vaccinated with BCG-iv (1 × 10⁶ CFU) for one month and mated with vaccinated or naive counterparts. 6–8 week-old F1.1 and F1.3 offspring were then vaccinated or not with BCG-iv (1 × 10⁶ CFU).
b), c) At 1 month post BCG vaccination, protective capacities of BMDM from BCG-iv vaccinated and nonvaccinated F1.1 (b), or F1.3 (c) offspring from naïve or BCG-iv vaccinated parents were assessed against M. tuberculosis (H37Rv, MOI 1) infection. * p < 0.05.”
https://www.nature.com/articles/s41590-021-01102-0 “Lack of evidence for intergenerational inheritance of immune resistance to infections” (not freely available)
Part 1 coauthors replied:
“We are very encouraged that this topic is gaining increased interest. The reason for the discrepancy between findings in the two studies is unclear. It likely involves local differences in mouse substrains, housing, diet, microbiome, infection models, or other factors.
These findings underscore the effect of environment on intergenerational inheritance of infection resistance. What these environmental factors are and how these factors are integrated with regards to intergenerational inheritance remains largely elusive at this time.
One intriguing possibility that needs to be tested in future studies is whether such effects may be more robust in outbred wild mice, in which subtle environmental changes may have less strong impact.”
https://www.nature.com/articles/s41590-021-01103-z “Reply to: ‘Lack of evidence for intergenerational inheritance of immune resistance to infections'”