This 2020 US human study investigated autism improvements with sulforaphane:
“Autism Spectrum Disorder (ASD) is one of the most common neurodevelopmental disorders that, in the United States, is currently estimated to affect 1 out of 59 children who are 8 years old. Despite decades of research and advances in our knowledge of etiologies of ASD, treatments and biomarkers for ASD remain limited.
The primary diagnosis of ASD still relies on observational tools that are by nature subjective. There are currently no drugs approved to treat core symptoms of ASD, nor are there any studies using SF [sulforaphane] in genetic mouse models of ASD.
In our previous placebo-controlled, double-blinded, randomized clinical trial, daily administration of SF for 4-18 weeks substantially improved behavioral abnormalities of the majority of 26 young males with moderate to severe ASD without significant toxicity. The multi-functional phytochemical sulforaphane affects many biochemical abnormalities associated with ASD.
We investigated potential molecular markers from three ASD-associated physiological pathways that can be affected by sulforaphane:
- Redox metabolism / oxidative stress;
- Heat shock response; and
- Immune dysregulation / inflammation
in peripheral blood mononuclear cells (PBMCs) from healthy donors and patients with ASD.
Three representative Nrf2 [nuclear factor erythroid 2-related factor 2]-dependent enzymes:
- AKR1C1 [aldo-keto reductase family 1 member C1];
- NQO1 [dehydrogenase quinone 1]; and
- HO-1 [heme oxygenase]
were significantly induced by 6 h of 2 μM or 5 μM SF ex vivo treatments in PBMCs from healthy donors. This time point was chosen based on our earlier observations of kinetics of upregulation of Nrf2-dependent genes by SF, and was expected to capture increased mRNA production of both very fast (HO-1) and relatively slow (NQO1) responders.
There was no concentration-dependence in induction of any genes examined, with higher (5 μM) concentration of SF even showing a slightly diminished effect for induction of AKR1C1 and NQO1. Although this concentration is achievable in vivo, more typical peak concentrations of SF (and its metabolites) in human plasma are 1-2 μM.
SF ex vivo pre-treatment significantly decreased the LPS [lipopolysaccharides]-stimulated inflammatory gene (
- COX-2,
- TNF-α,
- IL-6 and
- IL-1β
) expression levels in PBMCs from healthy donors.
As a pilot study for a clinical trial of SF in children with ASD, we evaluated the same biomarkers from the ex vivo studies in 10 young males with ASD, 6-12 years of age, who received SF (in the form of a dietary supplement containing GR [glucoraphanin] and myrosinase), 2.2 μmol/kg/d for 14 days. Grouping by broad functionality (e.g. cytoprotective or pro-inflammatory), differences from baseline were highly significant.
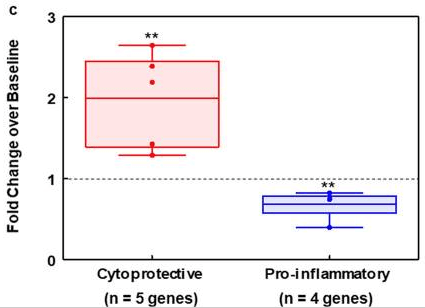
Individually none is sufficiently specific or sensitive, but when grouped by function as two panels, these biomarkers show promise for monitoring pharmacodynamic responses to sulforaphane in both healthy and autistic humans, and providing guidance for biomedical interventions. We conducted this study in the context of ASD, however our findings have broader implications and suggest that these biomarkers can be used in any study involving an intervention with SF.
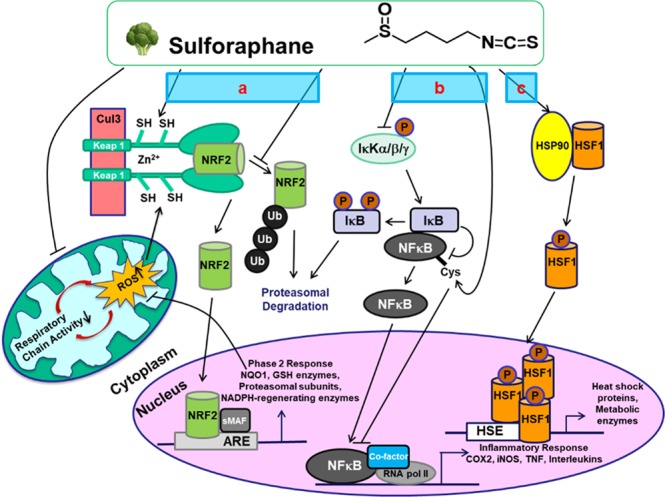
Major signaling pathways for protective mechanisms against ASD by SF:
- (a) Keap1/Nrf2/ARE pathway,
- (b) NF-κB inflammatory pathway,
- (c) Heat-shock responses.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118069/ “Biomarker Exploration in Human Peripheral Blood Mononuclear Cells for Monitoring Sulforaphane Treatment Responses in Autism Spectrum Disorder”
This was a pilot study. Does sulforaphane treat autism? was its follow-on clinical trial.
Broccoli sprouts and sulforaphane aren’t panaceas. Their research is becoming more intensive and focused, though.