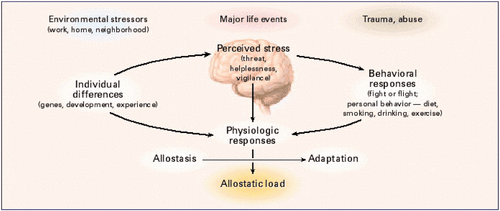
This 2018 US Veterans Administration review subject was resiliency and stress responses:
“Neurobiological and behavioral responses to stress are highly variable. Exposure to a similar stressor can lead to heterogeneous outcomes — manifesting psychopathology in one individual, but having minimal effect, or even enhancing resilience, in another.
We highlight aspects of stress response modulation related to early life development and epigenetics, selected neurobiological and neurochemical systems, and a number of emotional, cognitive, psychosocial, and behavioral factors important in resilience.”
The review cited studies I’ve previously curated:
- The truth about complex traits and GWAS that I curated yesterday;
- Conscious mental states should not be the first-choice explanation of behavior on the first day of this blog, February 1, 2015; and
- Manufacturing PTSD evidence with machine learning, but I had a different view of the study than the reviewers’ favorable one.
There were two things I didn’t understand about this review. The first was why the paper isn’t freely available. It’s completely paid for by the US taxpayer, and no copyright is claimed. I recommend contacting the authors for a copy.
The second was why the VA hasn’t participated in either animal or human follow-on studies to the 2015 Northwestern University GABAergic mechanisms regulated by miR-33 encode state-dependent fear. That study’s relevance to PTSD, this review’s subject, and the VA’s mission is too important to ignore. For example:
“Fear-inducing memories can be state dependent, meaning that they can best be retrieved if the brain states at encoding and retrieval are similar.
“It’s difficult for therapists to help these patients,” Radulovic said, “because the patients themselves can’t remember their traumatic experiences that are the root cause of their symptoms.”
The findings imply that in response to traumatic stress, some individuals, instead of activating the glutamate system to store memories, activate the extra-synaptic GABA system and form inaccessible traumatic memories.”
I curated the research in A study that provided evidence for basic principles of Primal Therapy. These researchers have published several papers since then. Here are the abstracts from three of them:
Experimental Methods for Functional Studies of microRNAs in Animal Models of Psychiatric Disorders
“Pharmacological treatments for psychiatric illnesses are often unsuccessful. This is largely due to the poor understanding of the molecular mechanisms underlying these disorders. We are particularly interested in elucidating the mechanism of affective disorders rooted in traumatic experiences.
To date, the research of mental disorders in general has focused on the causal role of individual genes and proteins, an approach that is inconsistent with the proposed polygenetic nature of these disorders. We recently took an alternative direction, by establishing the role of miRNAs in the coding of stress-related, fear-provoking memories.
Here we describe in detail our work on the role of miR-33 in state-dependent learning, a process implicated in dissociative amnesia, wherein memories formed in a certain brain state can best be retrieved if the brain is in the same state. We present the specific experimental approaches we apply to study the role of miRNAs in this model and demonstrate that miR-33 regulates the susceptibility to state-dependent learning induced by inhibitory neurotransmission.”
Neurobiological mechanisms of state-dependent learning
“State-dependent learning (SDL) is a phenomenon relating to information storage and retrieval restricted to discrete states. While extensively studied using psychopharmacological approaches, SDL has not been subjected to rigorous neuroscientific study.
Here we present an overview of approaches historically used to induce SDL, and highlight some of the known neurobiological mechanisms, in particular those related to inhibitory neurotransmission and its regulation by microRNAs (miR).
We also propose novel cellular and circuit mechanisms as contributing factors. Lastly, we discuss the implications of advancing our knowledge on SDL, both for most fundamental processes of learning and memory as well as for development and maintenance of psychopathology.”
Neurobiological correlates of state-dependent context fear
“Retrieval of fear memories can be state-dependent, meaning that they are best retrieved if the brain states at encoding and retrieval are similar. Such states can be induced by activating extrasynaptic γ-aminobutyric acid type A receptors (GABAAR) with the broad α-subunit activator gaboxadol. However, the circuit mechanisms and specific subunits underlying gaboxadol’s effects are not well understood.
Here we show that gaboxadol induces profound changes of local and network oscillatory activity, indicative of discoordinated hippocampal-cortical activity, that were accompanied by robust and long-lasting state-dependent conditioned fear. Episodic memories typically are hippocampus-dependent for a limited period after learning, but become cortex-dependent with the passage of time.
In contrast, state-dependent memories continued to rely on hippocampal GABAergic mechanisms for memory retrieval. Pharmacological approaches with α- subunit-specific agonists targeting the hippocampus implicated the prototypic extrasynaptic subunits (α4) as the mediator of state-dependent conditioned fear.
Together, our findings suggest that continued dependence on hippocampal rather than cortical mechanisms could be an important feature of state-dependent memories that contributes to their conditional retrieval.”
Here’s an independent 2017 Netherlands/UC San Diego review that should bring these researchers’ efforts to the VA’s attention:
MicroRNAs in Post-traumatic Stress Disorder
“Post-traumatic stress disorder (PTSD) is a psychiatric disorder that can develop following exposure to or witnessing of a (potentially) threatening event. A critical issue is to pinpoint the (neuro)biological mechanisms underlying the susceptibility to stress-related disorder such as PTSD, which develops in the minority of ~15% of individuals exposed to trauma.
Over the last few years, a first wave of epigenetic studies has been performed in an attempt to identify the molecular underpinnings of the long-lasting behavioral and mental effects of trauma exposure. The potential roles of non-coding RNAs (ncRNAs) such as microRNAs (miRNAs) in moderating or mediating the impact of severe stress and trauma are increasingly gaining attention. To date, most studies focusing on the roles of miRNAs in PTSD have, however, been completed in animals, using cross-sectional study designs and focusing almost exclusively on subjects with susceptible phenotypes.
Therefore, there is a strong need for new research comprising translational and cross-species approaches that use longitudinal designs for studying trajectories of change contrasting susceptible and resilient subjects. The present review offers a comprehensive overview of available studies of miRNAs in PTSD and discusses the current challenges, pitfalls, and future perspectives of this field.”
Here’s a 2017 Netherlands human study that similarly merits the US Veterans Administration’s attention:
“Posttraumatic stress disorder (PTSD) affects many returning combat veterans, but underlying biological mechanisms remain unclear. In order to compare circulating micro RNA (miRNA) of combat veterans with and without PTSD, peripheral blood from 24 subjects was collected following deployment, and isolated miRNA was sequenced.
PTSD was associated with 8 differentially expressed miRNA. Pathway analysis shows that PTSD is related to the axon guidance and Wnt signaling pathways, which work together to support neuronal development through regulation of growth cones. PTSD is associated with miRNAs that regulate biological functions including neuronal activities, suggesting that they play a role in PTSD symptomatology.”
See the below comments for reasons why I downgraded this review’s rating.
https://link.springer.com/article/10.1007/s11920-018-0887-x “Stress Response Modulation Underlying the Psychobiology of Resilience” (not freely available)