Two papers, starting with a 2023 study that investigated the same red radish cultivar as Sulforaphene, a natural analog of sulforaphane:
“Availability of microgreen products is constantly rising, i.e., they are offered for sale in local farmers markets, specialty stores, and in chain grocery stores. Due to the low demands required for their cultivation and easily available LED settings, microgreens are increasingly grown on a small scale in homes and after harvesting, they are stored in kitchen refrigerators at 4 °C.
The aim of this study was to simulate such cultivation and storage conditions to examine antioxidant capacity of home-grown radish microgreens. Seven-day-old radish microgreens, grown under purple and white LED light, were harvested and stored at 4 °C for two weeks.
Measurements of total antioxidant capacity and bioactive substances were conducted on the harvesting day and on the 3rd, 7th, and 14th day of storage. All three radish cultivars (Raphanus sativus L.) with different leaf colorations:
- Purple radish (R. sativus cult. China Rose, cvP);
- Red radish (R. sativus cult. Sango, cvR); and
- Green radish (Raphanus sativus var. longipinnatus, Japanese white or daikon radish, cvG)
were purchased commercially from a local supplier.
The highest contents of total soluble phenolics, proteins, and sugars, dry matter, and monomeric anthocyanin content, as well as higher overall antioxidant capacity determined in the red radish cultivar (cvR), distinguished this cultivar as the most desirable for human consumption regardless of the cultivation light spectrum.”
https://www.mdpi.com/2311-7524/9/1/76 “Antioxidant Capacity and Shelf Life of Radish Microgreens Affected by Growth Light and Cultivars”
A 2021 review summarized what was known about radishes up to then. Here’s part of its Discussion section:
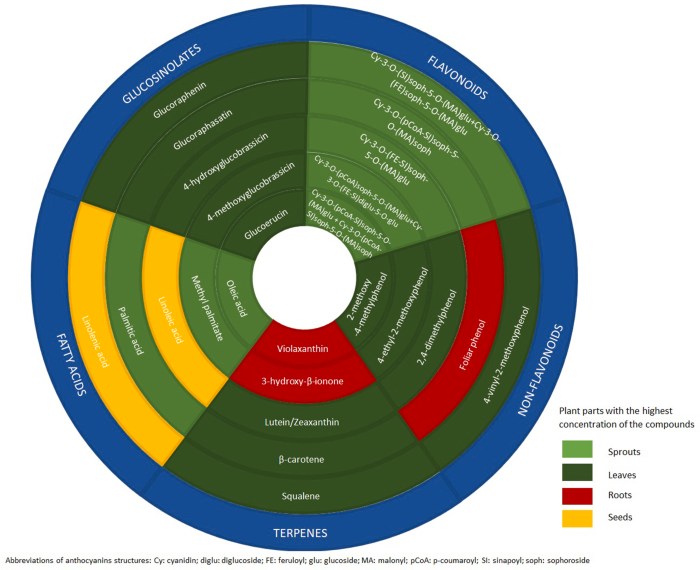
“It is worth considering radish’s organoleptic characteristics since its particular flavor can influence its acceptability among consumers. The main compound associated with its characteristic pungent flavor is raphasatin, which we have found to be the most reported isothiocyanate produced from the breakdown of glucoraphasatin.
Glucoraphasatin ranked as one of the most concentrated glucosinolates in radish, particularly in its sprouts, but also present in other parts like roots and seeds. Pungency differs among radish cultivars, environmental growth factors, agronomic, and cooking practices.”
https://www.sciencedirect.com/science/article/pii/S0924224421003058 “Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review”
Seeds I’ve sprouted this year so far, left to right – red radish (Sango), broccoli, red cabbage (Red Acre), yellow mustard, oat (Avena sativa):
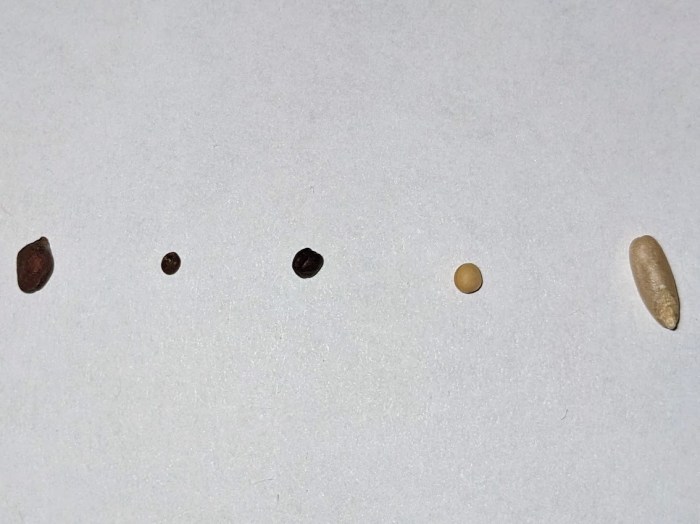
Red radish had similar growth characteristics as broccoli. Started with 3.6 grams of seeds, which increased to 22.2 g after three days using the same soaking and rinsing protocol I use for other sprouts.

The taste of red radish was too sharp for me to eat by themselves, so I combined them with my broccoli / red cabbage / mustard sprout mix. Bumped up microwaving time to 48 seconds in a 1000 W microwave while staying short of the 60°C (140°F) myrosinase cliff.
The whole mix still had a strong radish taste, though. It was as if two whole red radishes were sliced into a small salad.
Can’t add anything more to dampen that taste and expect beneficial compounds to be unaffected. From Week 19:
A 2018 Netherlands study Bioavailability of Isothiocyanates From Broccoli Sprouts in Protein, Lipid, and Fiber Gels found:
“Compared to the control broccoli sprout, incorporation of sprouts in gels led to lower bioavailability for preformed sulforaphane and iberin.”
IAW, eating protein, fats, and fiber along with microwaved broccoli sprouts wouldn’t help. A 2018 review with some of the same researchers Isothiocyanates from Brassica Vegetables-Effects of Processing, Cooking, Mastication, and Digestion offered one possible explanation for protein acting to lower broccoli sprout compounds’ bioavailability:
“In vitro studies show that ITCs can potentially react with amino acids, peptides, and proteins, and this reactivity may reduce the ITC bioavailability in protein‐rich foods. More in vivo studies should be performed to confirm the outcome obtained in vitro.”
Mixing in red radish sprouts also gave me an upset stomach five of the six mornings. So I won’t continue to sprout red radish.
That said, I’d definitely consider sprouting red radish again to accelerate isothiocyanate treatment of problems where symptoms are much worse than an upset stomach, such as:
- Neurogenerative diseases with their cognitive decline;
- Immune system disorders;
- Bacterial and viral infections; and
- Other damage caused by oxidative stress conditions in eyes, vascular system, kidney function, etc.
Piping in the New Year