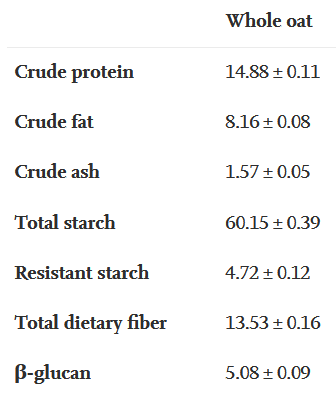
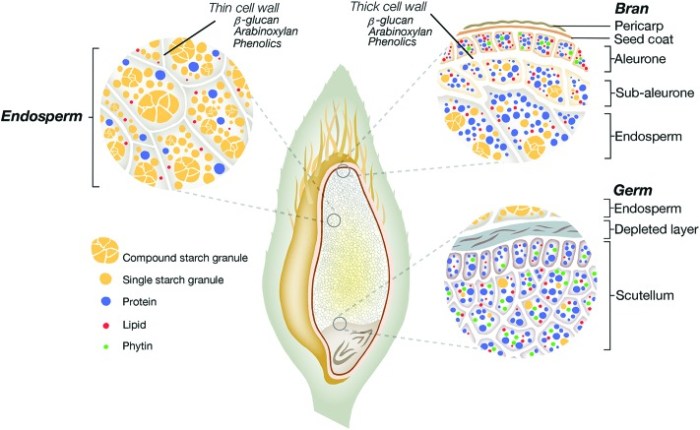
This 2020 study compared and contrasted distributional compositions of two oat species’ seeds:
“Oat grains of one hulless variety (Lamont) with low avenanthramide (AVA) contents and one hulled variety (Reins) with high AVA contents were sequentially abraded. Contents of nutrients (protein, oil, starch, β-glucan, ash, and other carbohydrates) and AVAs were measured.
A relationship between content of a constituent in the surface layer abraded off (termed pearling fine, or PF) at each cycle of pearling and the cumulative level of surface removal could be established. This relationship essentially describes true distribution or localization of individual constituents across an oat kernel.
AVAs provide health benefits in mammals, including anti-oxidation, anti-inflammation, anti-atherosclerosis, and anti-cancer properties. Relationships between contents of four AVAs and total AVAs in pearling fines (A) and corresponding pearled kernels (B) of hulless Lamont oat [top] and hulled Rein [bottom] with cumulative surface removal levels achieved by sequential pearling:
For Lamont oat, AVAs 2c, 2f, 2p, 5p, and total AVAs all showed decreasing concentrations with increasing levels of surface removal. The first PF (4% surface removal) contained the highest amounts for all four AVAs, with 2p near ten times higher than in whole grain.
Hulled Reins oat differed significantly from hulless Lamont oat in not only amounts of AVAs but also their distribution patterns within kernels. Dehulling caused reduction in total AVA content.
Pearled oats contained less protein, oil, ash, and other carbohydrates and AVAs, but more starch than whole grain. In contrast, oat bran contained more AVAs, protein, oil, ash and other carbohydrates but less β-glucan and starch as compared to whole grain.”
https://www.sciencedirect.com/science/article/abs/pii/S0308814620315302 “Distributions of nutrients and avenanthramides within oat grain and effects on pearled kernel composition” (not freely available)
There were higher AVA contents in hulls of the top graphic’s species (Avena nuda) compared with its next ten seed layers. Humans require the bottom graphic’s oat species’ (Avena sativa) hull, which is “about 25% total grain mass,” to be milled off before we eat it. So AVA data points on the bottom graph A start around 25% surface removal.
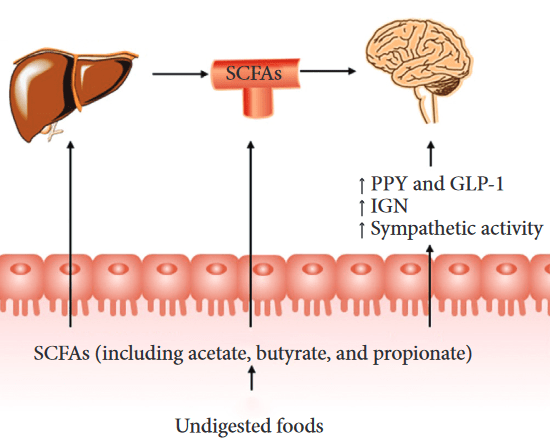
As mentioned in Eat oats to prevent diabetes, I replaced steel-cut Avena sativa oats with whole Avena nuda oats for breakfast. I don’t know how well Avena nuda hulls are digested, but gut microbiota ferment similar indigestibles into beneficial compounds.
The first study of Eat oat sprouts for AVAs found “up to 25-fold increase” in AVAs with 7-day-old Avena sativa sprouts. I expect 3-day-old hulled Avena sativa sprouts I eat also increase AVAs as they germinate.






 Sand sculptures
Sand sculptures
 Surface! Surface! Surface!
Surface! Surface! Surface!