Two epigenetic clock papers, starting with a 2022 rodent study:
“We tested performance of new pan-tissue and liver-specific epigenetic mouse clocks, evaluating how these related to metabolic states, genotype-dependent life expectancy, and methylome entropy.
Entropy, a measure of noise and information loss, increases as a function of time and age. In context of the methylome, higher entropy represents a tendency for the highly organized hypo- and hypermethylated landscape to erode towards a more hemi-methylated [discordant] state.
This increase in disorder, particularly across CpGs that are highly conserved, could have important functional consequences. Entropy of age-gain CpGs was increased by high fat diet, and predicted strain lifespan.
Overall, we find that mice belonging to longer-lived BXD strains had a more youthful methylome with lower entropy at age-gain CpGs. Entropy of age-loss CpGs on the other hand, was related to body weight.

(h) Residual plot (adjusted for age, diet, BWF [final body weight], glucose, cholesterol, and batch) shows an inverse association between entropy at age-gain sites, and lifespan. (i) A similar residual plot shows the association between BWF and age-loss entropy.
The rate of noise accumulation, an aspect of epigenomic aging, can vary between individuals. Resilience or susceptibility to higher noise may be partly modulated by diet as well as genetic factors.
Convergence of evidence from genetic and gene expression analyses indicates that genes involved in metabolism and energy balance contribute to age-dependent restructuring of the methylome, which in turn forms the basis of epigenetic clocks.”
https://elifesciences.org/articles/75244 “Genetic loci and metabolic states associated with murine epigenetic aging”
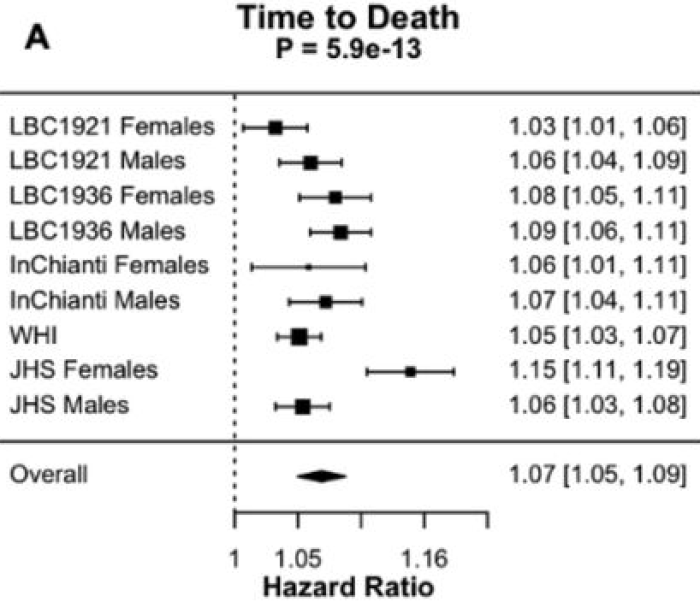
Reference 28 was a 2021 human study cited for “identified the APOE locus as the strongest GWAS hit for two measures of biological age acceleration”
“We observed inverse APOE e2 and e4 associations and unique pathway enrichments when comparing two biological age measures. Genes associated with BioAgeAccel were enriched in lipid related pathways, while genes associated with PhenoAgeAccel showed enrichment for immune system, cell function, and carbohydrate homeostasis pathways, suggesting the two measures capture different aging domains.
Our study reaffirms that aging patterns are heterogeneous across individuals, and the manner in which a person ages may be partly attributed to genetic predisposition. Understanding personalized aging susceptibility phenotypes has important implications for primary and secondary disease interventions.”
https://onlinelibrary.wiley.com/doi/10.1111/acel.13376 “Genetic associations for two biological age measures point to distinct aging phenotypes”