Three papers that cited the 2021 Reversing hair greying study, starting with a 2024 rodent study:
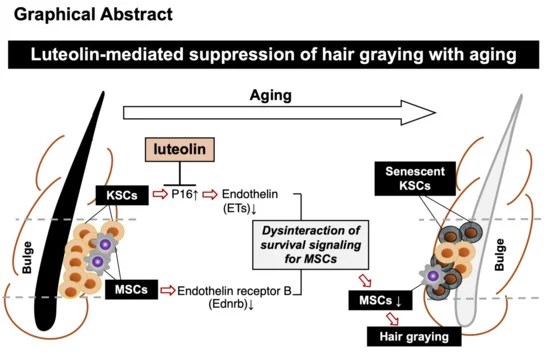
“External treatment with luteolin, but not that with hesperetin or diosmetin, alleviated hair graying in model mice. Internal treatment with luteolin also mitigated hair graying.
Both treatments suppressed the increase in p16ink4a-positive cells in bulges [senescent keratinocyte stem cells (KSCs)]. Both treatments also suppressed decreases in expression levels of endothelins in KSCs and their receptor (Ednrb) in melanocyte stem cells (MSCs), and alleviated hair graying in mice.”
https://www.mdpi.com/2076-3921/13/12/1549 “Anti-Graying Effects of External and Internal Treatments with Luteolin on Hair in Model Mice”
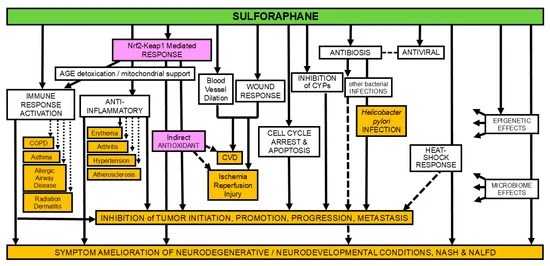
This study treated subjects internally and externally with luteolin and hesperetin, which are ranked #7 (effective treatment) and #14 (not an effective treatment) per Nrf2 activator rankings. I wonder what these researchers would have found if they used the #1 ranked Nrf2 activator, sulforaphane.
A 2024 review managed to cover the Nrf2 activation subject without mentioning sulforaphane:
“Certain types of hair graying can be prevented or treated by enhancing MSC maintenance or melanocyte function, reducing oxidative stress, and managing secretion and action of stress hormones.
Tactical approaches to pursue this goal may include a selective activation of the p38 MAPK–MITF axis, enhancing cellular antioxidant capacity through activating NRF2, and modulating the norepinephrine–β2AR–PKA signaling pathway.”
https://www.mdpi.com/2076-3417/14/17/7450 “Intrinsic and Extrinsic Factors Associated with Hair Graying (Canities) and Therapeutic Potential of Plant Extracts and Phytochemicals”
This reviewer also avoided citing the 2021 Sulforaphane and hair loss, although hair loss was mentioned multiple times. I suspect that institutional politics was involved, as both papers are from South Korea.
Reference 32 of this review was a 2023 review that covered mainly unintentional hair greying reversal as a side effect noted when people had pharmaceutical treatments for various diseases:
“Hair graying is a common and visible sign of aging resulting from decreased or absence of melanogenesis. It has long been thought that reversal of gray hair on a large scale is rare. However, a recent study reported that individual gray hair darkening is a common phenomenon, suggesting the possibility of large-scale reversal of gray hair.
All these treatments rely on the presence of a sufficient population of active McSCs. Maintaining a healthy population of McSCs is also an urgent problem that needs to be addressed.”
https://www.ijbs.com/v19p4588.htm “Reversing Gray Hair: Inspiring the Development of New Therapies Through Research on Hair Pigmentation and Repigmentation Progress”
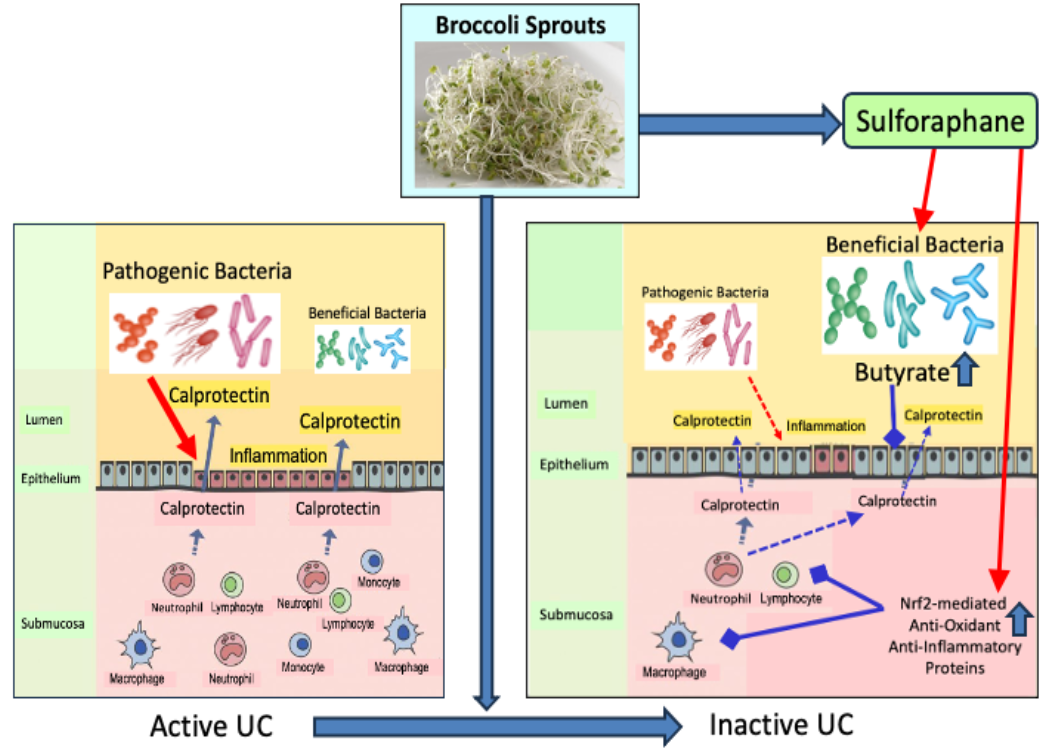
I published A hair color anecdote two months into eating broccoli sprouts every day when I first noticed dark hair growing in. Since it’s been over 4 years that I’ve continued eating broccoli sprouts daily, I think it’s alright to stop referring to my continuing reversal of hair greying as an anecdote.
But it was apparently too late to address hair loss, which started before I turned 30. So now you know what to do. 🙂