Three more 2023 papers that cited Precondition your defenses with broccoli sprouts, starting with a review:
“Examining crosstalk between Nrf2 antioxidant signaling and autophagy provides insights into how they are interconnected and proteins that mediate their communication. These factors are potential therapeutic targets for diseases with both autophagy dysfunction and oxidative stress.
A working model illustrates mechanisms of bridging factors (SQSTM1, TFEB, Sestrin2, TRIM16, Ca2+, and miRNAs) connecting autophagy (left) and the main antioxidant Nrf2-Keap1-ARE pathway (right) and feedback loops between these factors.
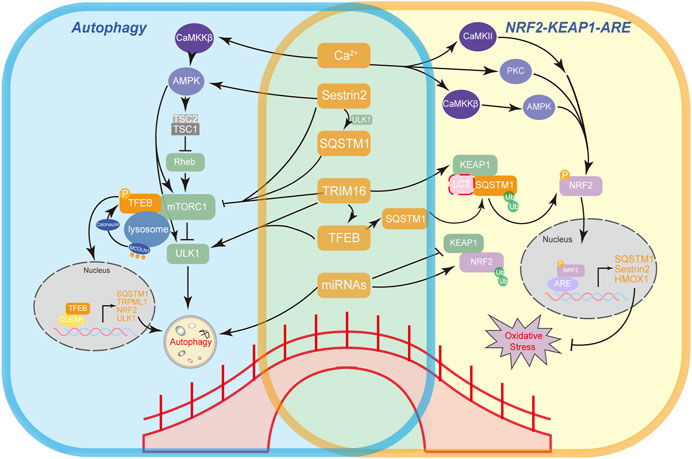
- A network forms that connects Nrf2, SQSTM1, TFEB, and mTOR.
- Other non-canonical autophagy regulatory proteins like Sestrin2 and tripartite motif-containing protein 16 (TRIM16) also participate in regulation of Nrf2 and mTOR via direct or indirect interactions.
- Ca2+ is the most widespread intracellular messenger whose role in autophagy has been studied extensively.
- At post-transcriptional level, microRNAs have been reported to impact both the regulation of autophagy and Nrf2 antioxidant signaling.
Since these regulatory proteins seem intricately entangled, potential side effects in practical scenarios should also be taken into consideration. Further studies on understanding the complex crosstalk between autophagy and antioxidant pathways are yet to be conducted.”
https://www.frontiersin.org/articles/10.3389/fcell.2023.1232241/full “An update on the bridging factors connecting autophagy and Nrf2 antioxidant pathway”
A second review subject was improving autophagy:
“Lysosomes are crucial degradative organelles that maintain cellular homeostasis. During the pathogenesis of neurodegenerative diseases and aging, functions of lysosomes are impaired, and lysosomal degradative capacity is consequently reduced.
Transcription factor EB-mediated lysosome biogenesis enhances autolysosome-dependent degradation, which subsequently alleviates neurodegenerative diseases. Small-molecule compounds that enhance TFEB activity and lysosome biogenesis are potential therapeutic agents.”
https://journals.lww.com/nrronline/fulltext/2023/11000/enhancement_of_lysosome_biogenesis_as_a_potential.7.aspx “Enhancement of lysosome biogenesis as a potential therapeutic approach for neurodegenerative diseases”
A third review tied mitochondrial participation into these processes:
“Mitochondria play an essential role in neural function, such as supporting normal energy metabolism, regulating reactive oxygen species, buffering physiological calcium loads, and maintaining the balance of morphology, subcellular distribution, and overall health through mitochondrial dynamics. Given recent technological advances in the assessment of mitochondrial structure and functions, mitochondrial dysfunction has been regarded as the early and key pathophysiological mechanism of cognitive disorders.
Mitochondrial dysfunction caused by acute and chronic brain injury is difficult to be distinguished because they may exhibit similar structural and functional impairments. Mitochondrial physiological function and morphology are integral, so when one is damaged, the other is also involved.
We recommend that all of the above methods can be used to explore mitochondrial dysfunction in different pathological pathways of cognitive disorders. Results may be related to special pathological pathways, sensitivity of the method, experiment cost, and degree of proficiency.”
https://journals.lww.com/nrronline/fulltext/2024/04000/latest_assessment_methods_for_mitochondrial.18.aspx “Latest assessment methods for mitochondrial homeostasis in cognitive diseases”
