Here’s a 2025 interview with Dr. Greg Fahy:
“We found that we could statistically demonstrate thymic regeneration morphologically on single individuals at single time points. MRI changes really are detecting shifts from the fatty tissue infiltration state of the involuted thymus to the regenerated thymus with functional thymic epithelial cells.
When you go through puberty your thymus involutes so you don’t have much left even when you’re 40. Essentially the process consists of loss of functional thymic mass and replacement of that functional thymic mass with adipose tissue, that’s what thymic involution is. It continues throughout life, but you retain a small amount of functional thymic mass all the way out to the age of 107.
The function of the thymus is to essentially manufacture half of your immune system. You have precursor cells arise from the bone marrow. They either go into the meiotic lineage and turn into the innate immune system, or you have the lymphocytic cells for what turns into T cells that enter the thymus and are educated in the thymus to grow up into newborn T cells and they’re released into the bloodstream.
The thymus has two jobs. It manufactures these lovely T cells without which you die but it also has a secondary finishing school. In the thymus cortex you manufacture all these lovely T cells but in the thymus medulla the T cells go to the medulla and if they don’t pass the second examination that they have to pass before they release into the body they’re all killed off. That second examination is: Do you reject self? As we get older, the thymus weakens in both the functions of making the T cells and screening out the ones that attack self. It stands to reason as we get older and the thymus’ influence wanes, we’re going to get more autoimmune disorders.
It took people a while to catch on to the fact that this involution problem is really a significant issue because the T cells that you made when you were 12, and even 20 and 40, they’re probably lasting until you’re 60. But at some point they don’t get replaced as fast as they’re going out of existence, and then your immune system goes off the cliff. Between the ages of 62 and 78 you lose 98% of your ability to recognize foreign antigens, and you still have a lot of capacity left.
We had nine guys in the first trial. Second trial we had 18 men 6 women and 2 controls that happen to be contemporaneous with that group. We have some more controls now that are either finished or or nearing completion. The second population was older than the first population by about nine years, but based on the epigenetic clocks that we looked at, they were starting off biologically younger.
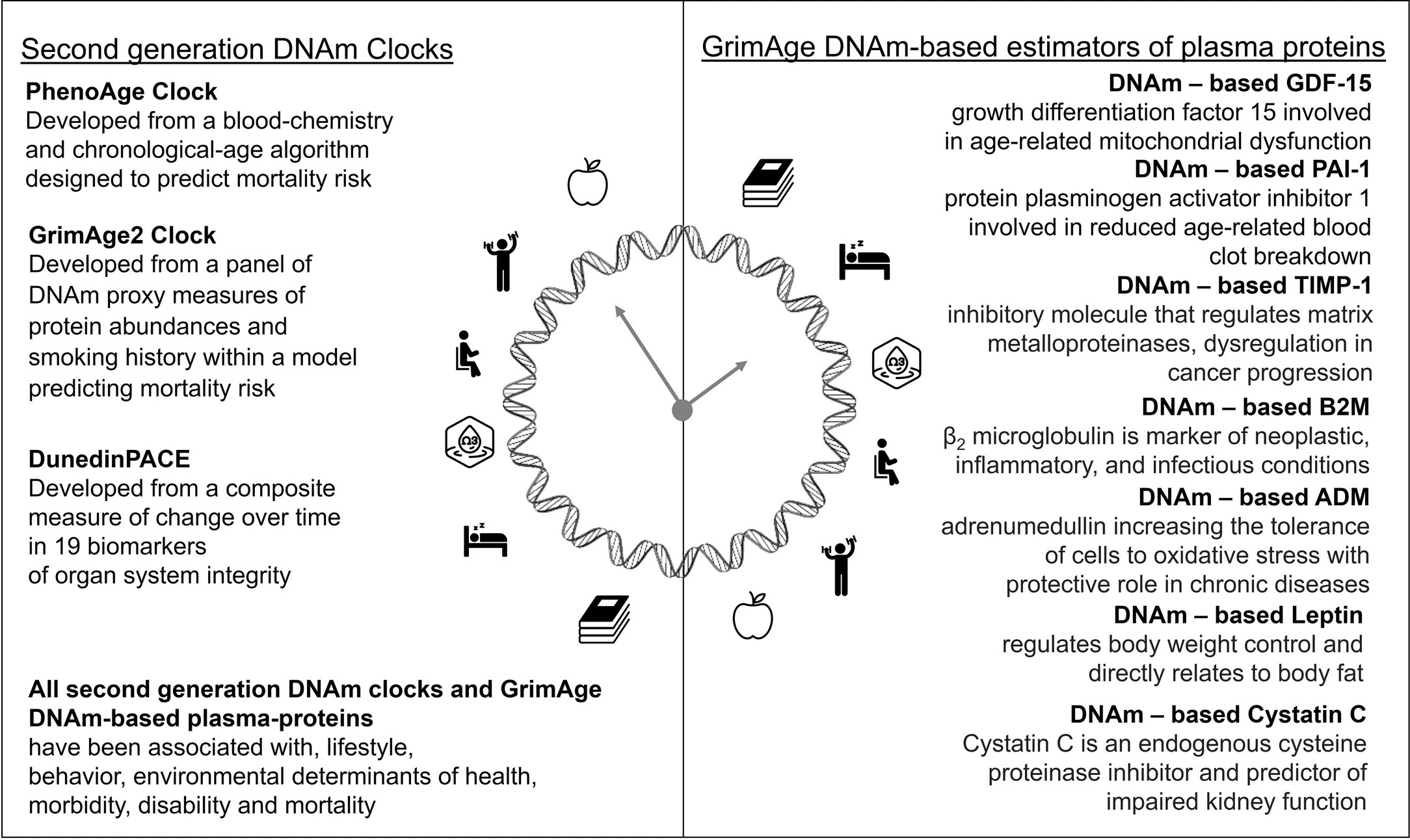
On this last data analysis for Triim XA we looked at 21 different aging clocks. One aspect of the noise that we’re talking about is that biological aging as measured by some of these clocks is circadian. If you measure your age at 4:00 a.m. versus 11:00 a.m. you’re going to get a different result. It’s dynamic and there’s a trend and over time you change in a certain direction, but over any short period of time you can bounce around a little bit. The clocks predict your probability of cognitive dysfunction, they predict your probability of having impairments in your daily life, and they also predict your mortality.
We’re pretty much wrapping up that second clinical trial and going into the third. As we look at more data we understand more and more things and we see more and more things that we previously were not aware of. We began to look at a phenomena that may be responsible for limiting the magnitude of responses that we’re seeing limiting the aging reversal.
Triim-XD which is the next flavor of Triim-X is going to be looking at shifting biochemical pathways in such a way that it optimizes effects of these three medications that we’re giving people [human growth hormone, DHEA, and metformin] and prevents contradictions between them and prevents side effects of each one of these things. That’s about all I can tell you right now.”
Charts regarding the discussed item of how long effects may last are covered in The next phase of reversing aging and immunosenescent trends which was the last time I curated this research effort.