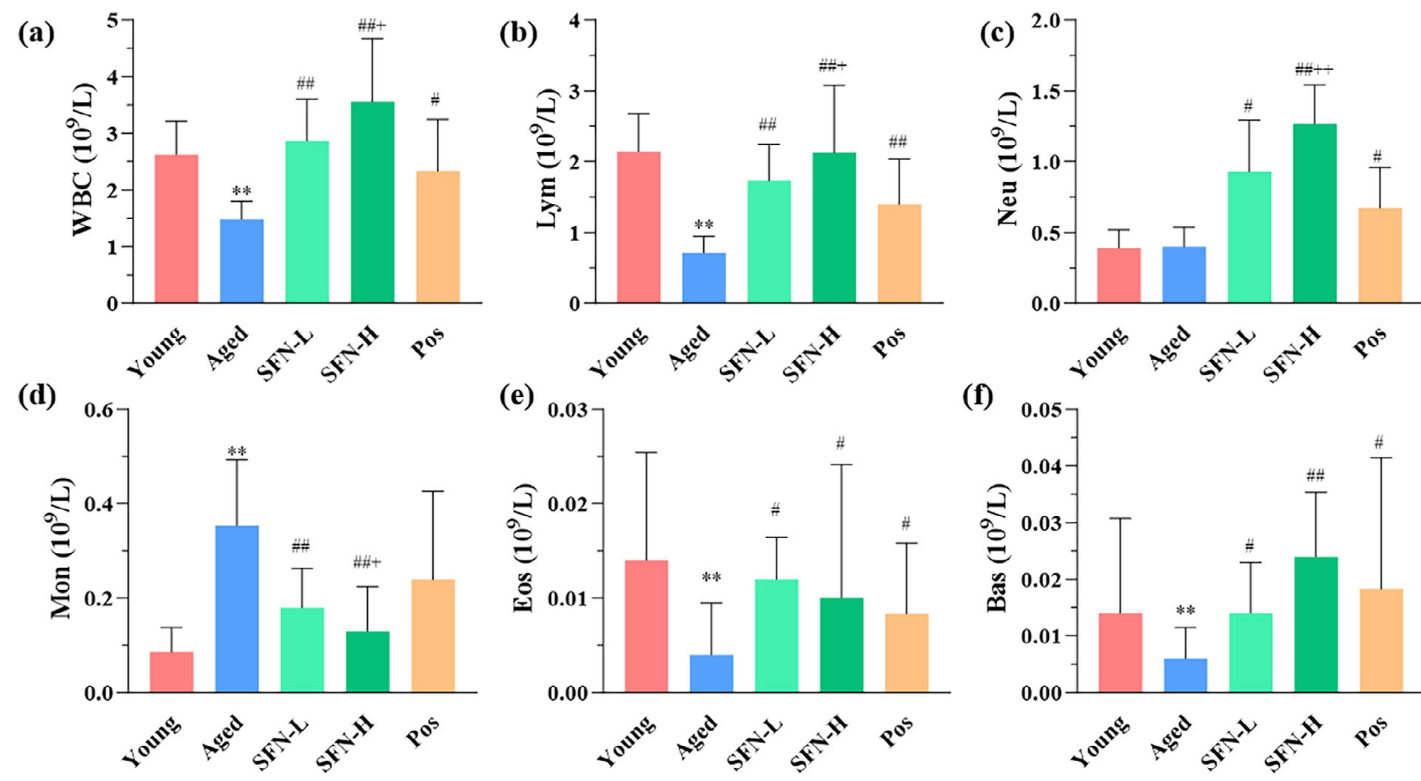
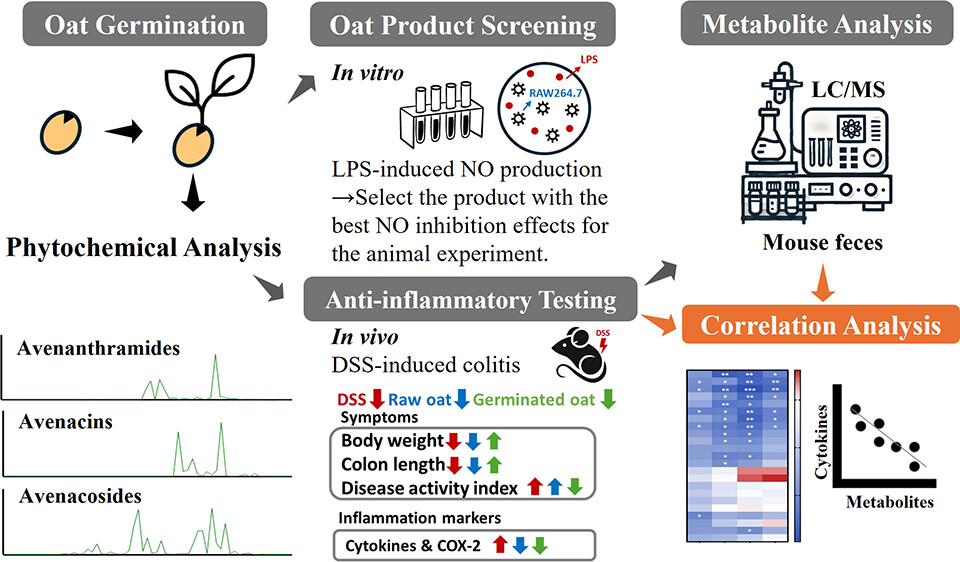
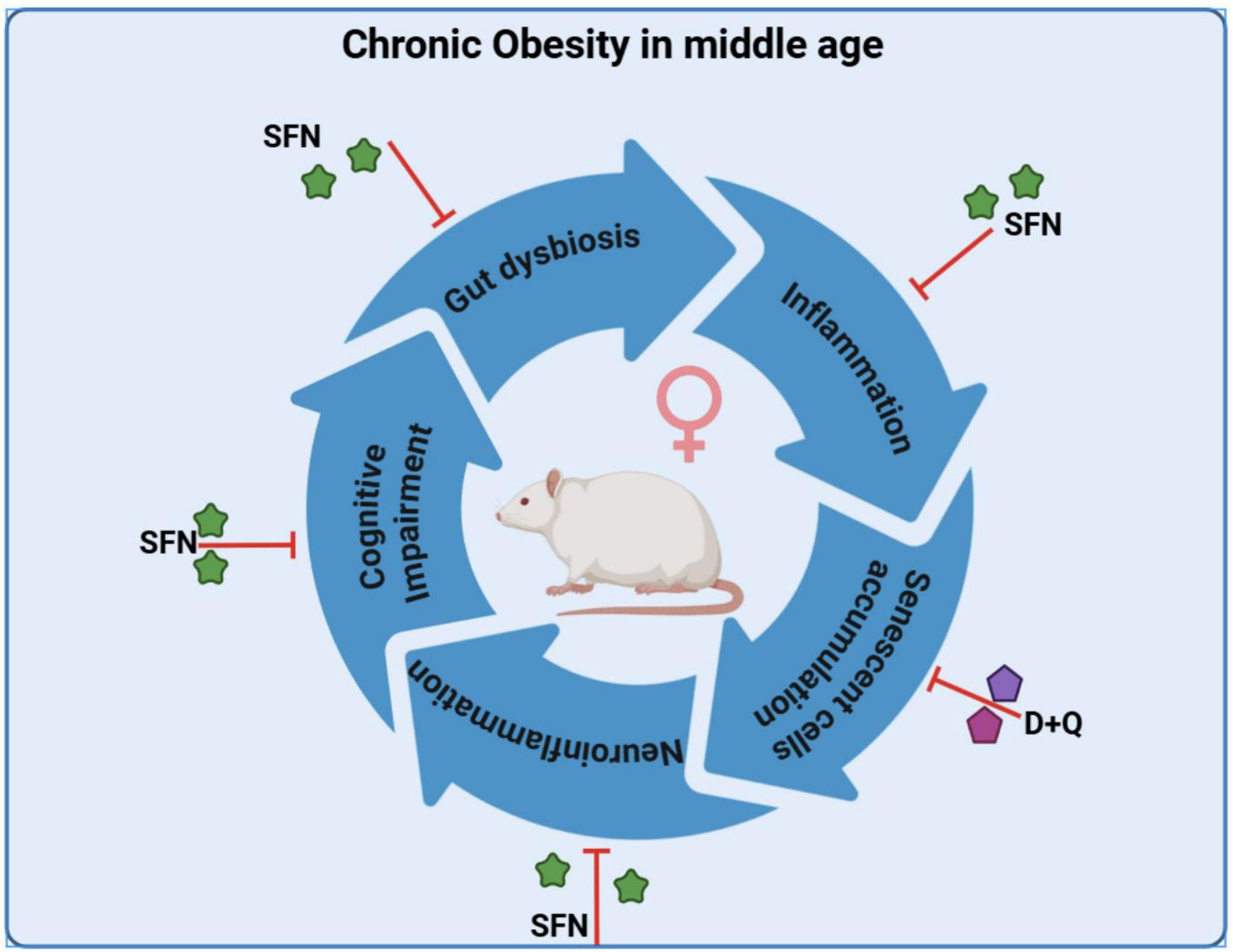
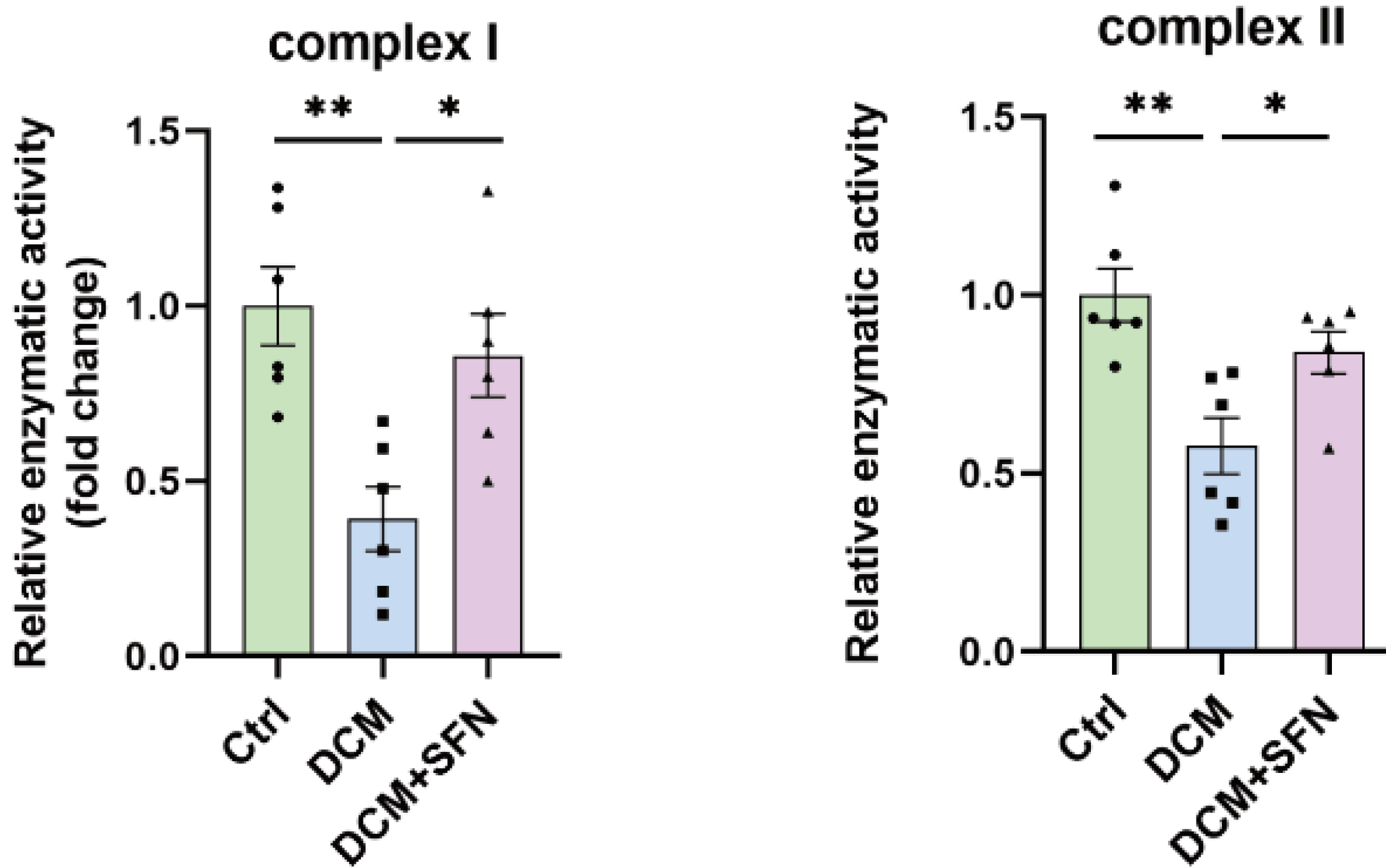
A 2025 rodent study investigated sulforaphane’s capability as an adjunct with standard treatment to inhibit resistant malaria strains:
“In this study, we performed proteomic analysis on a range of sensitive and artemisinin-resistant parasites, revealing specific dysregulation of PfK13 protein abundance. Reduced PfK13 levels were linked to impaired hemoglobin digestion, decreased free heme levels, and consequently, decreased artemisinin activation. Artemisinin resistant parasites also exhibited elevated thiol levels, indicating a more reduced cellular state.
Modulation of PfK13 levels or localisation modifies glutathione (GSH) levels, and elevated GSH decreases artemisinin potency. Elevated levels of reduced GSH and its precursor γ-glutamyl cysteine (gGlu-Cys) were observed in resistant parasites, while oxidised glutathione (GSSG) was lower.
In mammalian cells, SFN conjugates GSH, either passively or through the activity of glutathione-S-transferases, and the SFN-GSH conjugate causes oxidative stress. In response to this stress, Nrf2 translocates to the nucleus and interacts with the antioxidant response element (ARE) of target genes, resulting in expression of antioxidant genes, which induces an antioxidant response. However, P. falciparum has no identified Nrf2 orthologue and so likely lacks a KEAP1-Nrf2 mediated antioxidant response, which suggests that the SFN-GSH conjugate should only cause oxidative stress in parasites.
SFN has antioxidant properties for the host through activation of Nrf2. Therefore our molecule of choice would not only kill the parasite, but will boost the host antioxidant capacity. This differs from most other available pro-oxidants, which do not have this host antioxidant capacity.
5mg/kg SFN was found to be sufficient to significantly prolong the survival of artesunate-treated mice infected with parasites.
PfK13 mutations drive artemisinin resistance in Plasmodium parasites by enhancing antioxidant defences, which can be targeted by redox modulators such as sulforaphane. By leveraging SFN’s ability to induce oxidative stress and deplete thiol levels in parasites, this approach can enhance the efficacy of artemisinin and potentially restore its effectiveness against resistant strains.”
https://www.biorxiv.org/content/10.1101/2025.10.05.680568v1.full “PfK13-associated artemisinin resistance slows drug activation and enhances antioxidant defence, which can be overcome with sulforaphane”