A 2023 review cited Part 1 and caught up other relevant research on sulforaphane effects through early 2023:
“A growing number of studies have reported that sulforaphane (SFN) could significantly ameliorate hepatic steatosis and prevent development of fatty liver, improve insulin sensitivity, attenuate oxidative damage and liver injury, induce apoptosis, and inhibit proliferation of hepatoma cells through multiple signaling pathways.
SFN inhibits lipogenesis and oxidative stress while enhancing lipid droplet degradation through modulating expression of genes involved in lipid synthesis, metabolism, and oxidation. SFN modulates autophagy, lipolysis, mitochondrial function, and ER stress to alleviate fatty liver through AMPK-, AHR-, PGC1α-, and FGF21-mediated pathways.
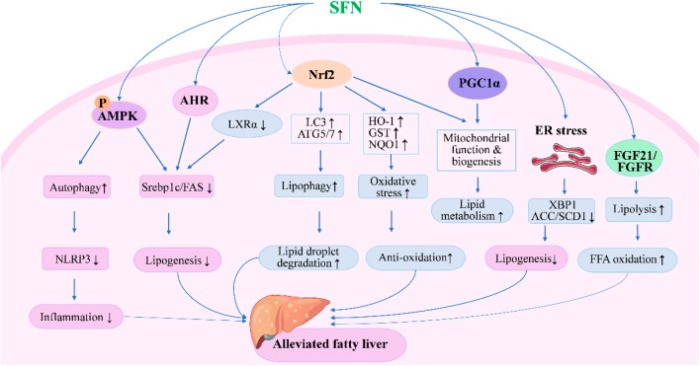
There is still a gap between basic research and clinical application of SFN. More efficient delivery systems and precise dose schedules of SFN are expected to be developed in future studies, which would improve its solubility, stability, and bioavailability, and reduce inter-individual variations in humans.”
https://www.frontiersin.org/articles/10.3389/fphar.2023.1256029/full “Therapeutic potential of sulforaphane in liver diseases: a review”
These reviewers did alright gathering papers. That’s only part of what needed to be done, with the other part being reading, understanding, and interpreting these papers.
First example: Sulforaphane in the Goldilocks zone was cited [reference 12], but applicability to this review with its main point “The stimulatory zone for in vitro studies proved to be consistently in the 1-10 μM range” as in Figure 10 “Effects of R-sulforaphane on phase II enzyme activation in precision-cut liver slices of young adult male Albino Wistar rats” wasn’t understood:
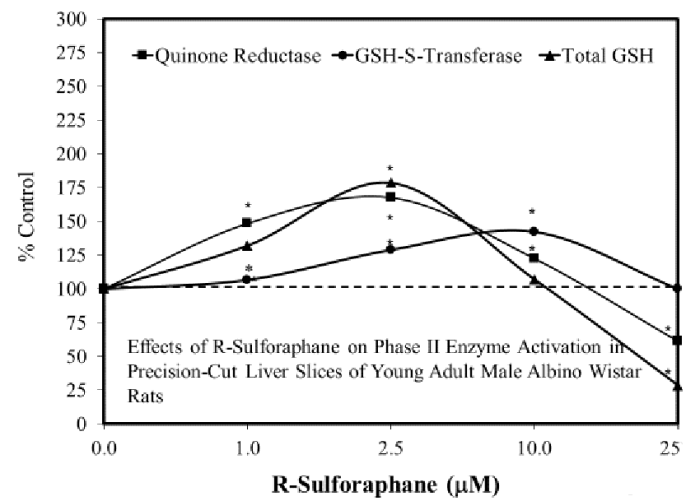
These reviewers complained:
“Few dose-response studies on SFN have been reported, and the range of its effective doses is unclear. Doses used in most animal studies have exceeded the highest dose of SFN used in humans.”
So it might have taken a little bit more effort, but these reviewers could have highlighted studies where sulforaphane liver treatments were in the 1-10 μM potentially therapeutic range.
Another example: these reviewers said “The half-life of SFN is very short due to its rapid metabolism in the human body.” They missed a point that the second paper in How much sulforaphane is suitable for healthy people? [reference 46] made in section 6.4. “NQO1 Pharmacokinetics following SFN Ingestion:”
“Maximal induction of NQO1 occurred at around 24 hours, declining thereafter (Figure 8). This peak represents an approximate 2.8-fold induction over baseline.
These findings are useful when considering the effect of SFN as an intervention material in acute compared with chronic conditions. A significant increase in NQO1 occurred between 6 and 12 hours, a timeframe that may not be sufficiently responsive for management of an acute state, leaving one to conclude that NQO1 induction is best suited to chronic conditions where a rapid response may not be necessary.”
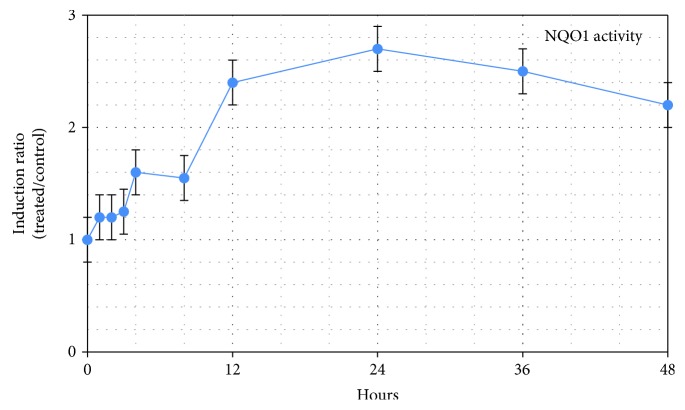
Sulforaphane’s effects of inducing NQO1 for its cytoprotective, antioxidant, and other functions lasts for days, regardless of when sulforaphane leaves the bloodstream.