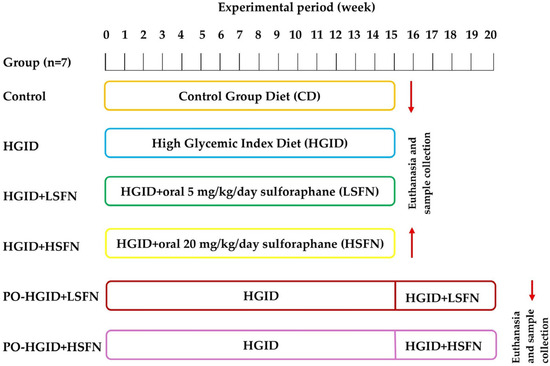
This 2026 rodent study made mice obese with a high-glycemic-index diet, and intervened with different doses of sulforaphane during and after inducing obesity:
“To our knowledge, this is the first study investigating therapeutic effects of sulforaphane (SFN) on obesity resulting from feeding with high-glycemic-index diet (HGID). To evaluate the potential role of SFN on energy metabolism, obesity development, and insulin resistance, the effects were tested by administering SFN at different doses [oral 5 mg and 20 mg] in addition to HGID and after animals were made obese with HGID.
The energy, macronutrient, and fiber contents of the HGID used in the experiment and the isocaloric control group feed were kept equal. The only difference between the HGID and the control group feed was the composition of the starch. While the starch in the HGID was a waxy corn starch consisting of 100% amylopectin, it was natural starch (75% amylopectin, 25% amylose) in the control group.
This study is strengthened by its:
- Experimental design, in which SFN was administered at multiple doses both during exposure to the HGID and after the development of HGID-induced obesity, allowing for a comprehensive evaluation of its effects on energy metabolism, obesity progression, and insulin resistance.
- Focus on the specific components of the HGID. To be able to separate the effects of the HGI diet pattern, one of the long-standing criticisms regarding GI, from individual components it contains, especially dietary fiber, we were able to evaluate the glycemic index interaction by keeping the energy, macronutrient, and fiber contents of the feeds equal and the starch composition different.
Several limitations should be acknowledged.
- Potential adverse effects of the 5 mg/kg/day and 20 mg/kg/day doses of sulforaphane in this study were evaluated in terms of clinical signs. However, systemic adverse effects, particularly those affecting the brain, cardiovascular system, or other organs, were not assessed.
- The relatively short duration of the SFN intervention (five weeks following the development of obesity induced by a HGID) may have limited the ability to fully capture all potential changes in the measured variables. It may be beneficial to observe for a longer period in future studies to provide evidence that SFN reverses HGID-induced obesity.
- The ideal dose of SFN has not yet been determined. Dose and bioavailability are considered important parameters that need to be clarified for SFN to be considered as an anti-obesity agent.
Results indicate that SFN may provide potential benefits both as a protective agent in the obesity development process and as a therapeutic approach after obesity has developed.
- While SFN suppresses obesity development by combating increased energy consumption, body weight, deteriorated lipid profile, and decreased insulin sensitivity upon exposure to HGID, it supports obesity treatment with its aspects of reducing food consumption and body weight gain and improving glycemic control.
- SFN may reverse the adverse effects of HGID in a time- and dose-dependent manner by regulating postprandial insulin, restoring IRS1/IRS2 function, inhibiting gluconeogenesis through the coordinated activation of signaling between sirtuins and PGC-1α, and shifting liver metabolism from lipid synthesis toward mitochondrial oxidation.”
https://www.mdpi.com/2072-6643/18/4/574 “Sulforaphane Against the Metabolic Consequences of a High-Glycemic-Index Diet: Protective and Therapeutic Mechanisms Associated with Obesity and Insulin Resistance”
A human equivalent to this study’s daily oral low sulforaphane dose is (5 mg x .081) x 70 kg = 28 mg, which is achievable by eating broccoli sprouts every day. People won’t tolerate quadrupling 28 mg to a human equivalent of the study’s 20 mg daily oral sulforaphane dose, so I didn’t curate this study’s high-sulforaphane-dose-specific findings.
Human age equivalents to this study’s 8-week-old, 23-week-old, and 28-week-old mice are respectively 18-25 years, 25-35 years, and 28-38 years.