A 2024 rodent study investigated epigenetic effects of loosening compacted chromatin:
“We show using a novel mouse strain, (TKOc), carrying a triple knockout of three methyltransferases responsible for H3K9me3 deposition, that the inducible loss of H3K9me3 in adulthood results in premature aging. TKOc mice exhibit:
- Reduced lifespan;
- Lower body weight;
- Increased frailty index;
- Multi-organ degeneration;
- Transcriptional changes with significant upregulation of transposable elements; and
- Accelerated epigenetic age.
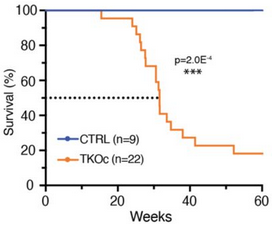
Through simultaneous depletion of Setdb1 and Suv39h1/2 methyltransferases, crucial to formation of constitutive heterochromatin, our model analyzes consequential transcription changes including a potential source of genomic instability by activation of endogenous mobile genetic elements, specifically transposable elements.
These findings reveal the importance of epigenetic regulation in aging, and suggest that interventions targeting epigenetic modifications could potentially slow down or reverse age-related decline.”
https://www.biorxiv.org/content/10.1101/2024.07.24.604929v1.full “Loss of H3K9 trimethylation leads to premature aging”
Many of these findings could be restated without viewing them as age-related, i.e.: failure to maintain an adult’s methyltransferase system results in a loss of health. For example, an unhealthy methyltransferase system indicated by parameters like homocysteine levels (not mentioned) can be reversed to healthy function regardless of age. Healthy vs. unhealthy system function wasn’t the paradigm these researchers operated under, though.
